This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Science (applied)
Science (applied)
1. Enzymes are proteins.
Most enzymes are globular proteins. The bonds holding the amino acids together are peptide bonds but hydrogen bonds, disulfide bonds and ionic bonds work together to produce a secondary and tertiary structure.
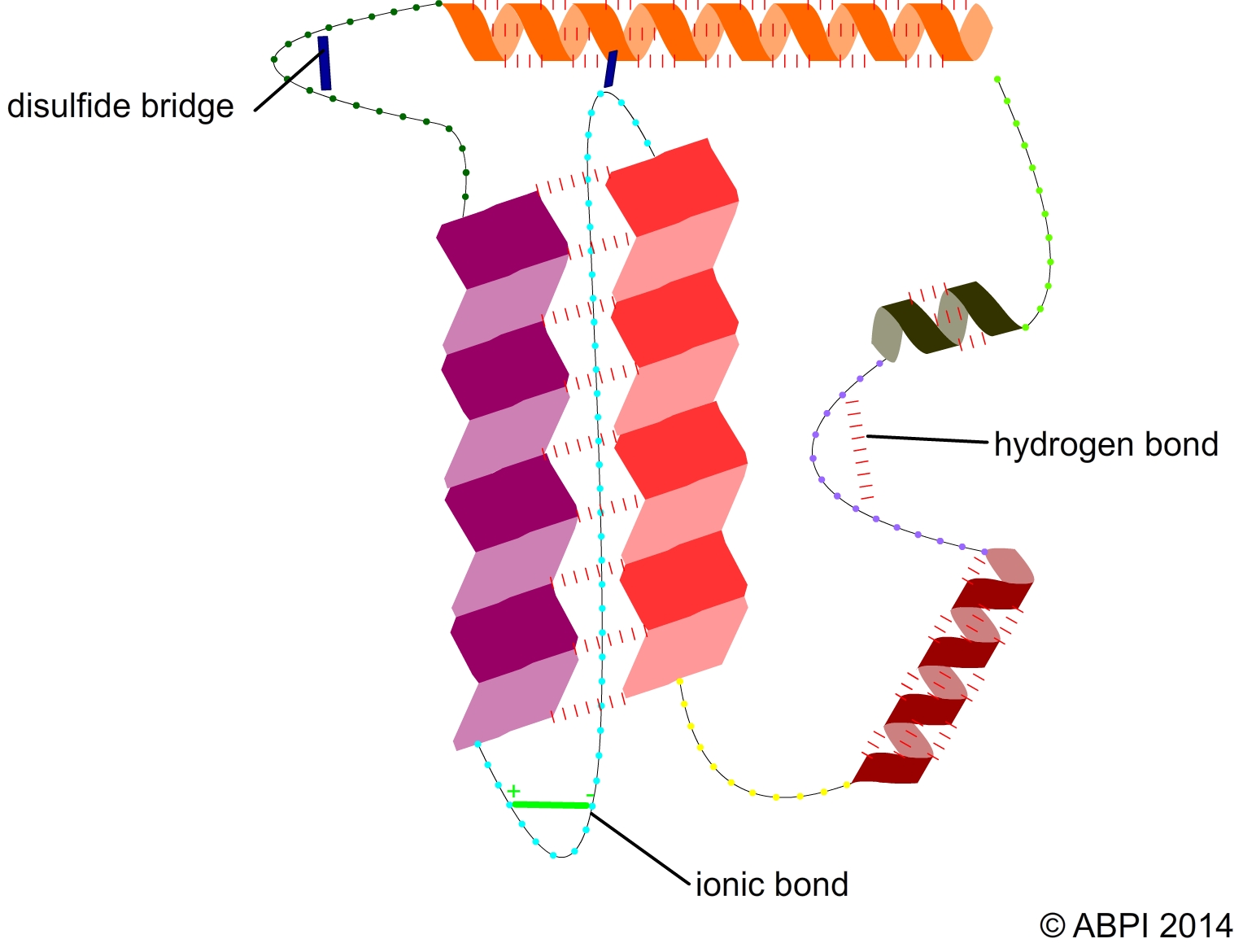
Most enzymes are globular proteins.
2. Enzymes have an active site.
Within the globular protein structure of an enzyme is the active site. This is a 3D depression or hollow shape that is vital to the way the enzyme functions. The three dimensional, stereospecific shape of the active site is the result of the folding of the protein molecule. Anything that affects the shape of the active site will affect the ability of the enzyme to bind to the substrate or substrates and catalyse a reaction.
3. Enzymes are very specific.
An enzyme will only catalyse one type of reaction. Some enzymes are so specific that they will only catalyse one particular reaction. This is due to shape of the active site. The active site is stereospecific – in other words, it is specific in three dimensions. So, for example, it will only bind to one stereoisomer or enantiomer of a substrate molecule, not both (see Chemistry of Life).
4. Enzymes change the rate of a reaction.
They act as catalysts so they do not affect the end products or the equilibrium of the reaction that they catalyse.
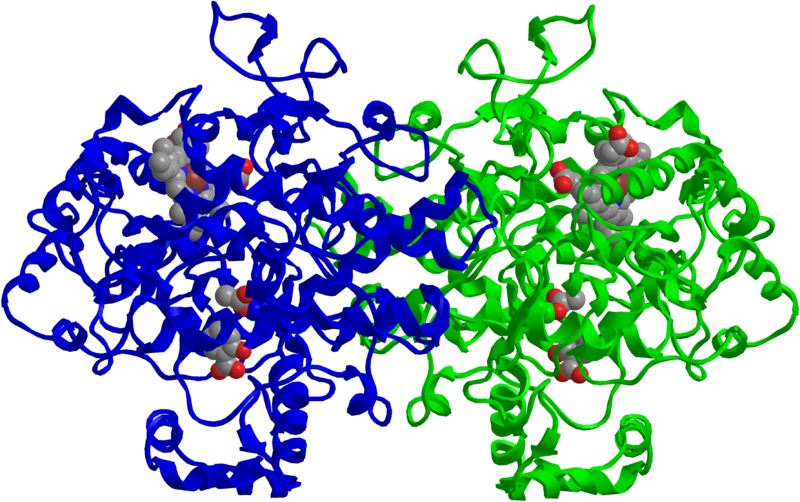
This computer generated model of the enzyme COX-2 shows the active site in red (Jeff Dahl, public domain).
From Roman times to the Victorian era, dog faeces and pigeon droppings were used in the tanning process to help produce supple leather. This is the earliest – and perhaps the most unpleasant – example of people using enzymes in industry. At the very end of the 19th century, the German chemist Otto Rohm discovered that it was protein-digesting enzymes in the faeces that had the desired effect on the leather. By 1905 he had developed a way of producing proteases to soften leather from cow and pig pancreases – undoubtedly a great relief to leather workers everywhere!
In 1835, people noticed that starch was hydrolysed faster by malt than by sulphuric acid. This led to the idea that there was an catalyst present in living malt that was more effective than the inorganic acid.
People suspected there was a biological catalyst in yeast which brought about the fermentation of sugar to alcohol long before anyone proved it. In 1877, people started to use the name enzyme (literally 'in yeast') for these theoretical chemicals. In 1897, Eduard Buchner extracted the enzyme responsible for fermenting sugar from yeast cells, and showed it could work independently of the living cell structure.

Animal faeces contain enzymes that were used for centuries to soften leather
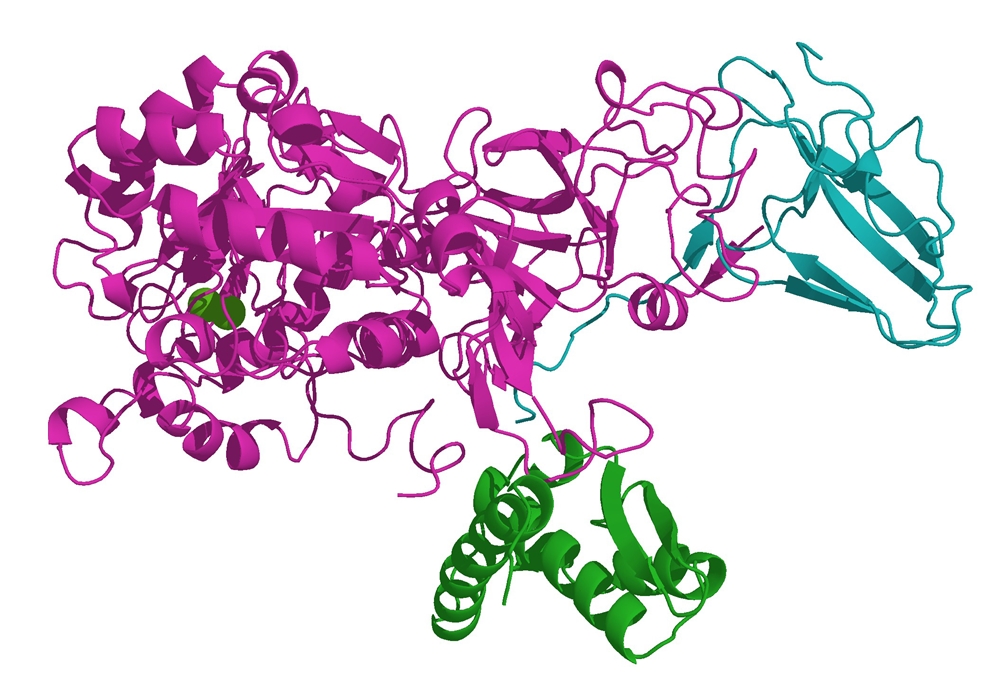
A computer generated model of urease (Ayacop, public domain)
The first pure, crystalline enzyme was produced in 1926 by Sumner. It was the enzyme urease, that catalyses the breakdown of urea. He extracted it from jack beans. Sumner showed that the crystals were protein and concluded that enzymes must be proteins. Unfortunately no-one believed him to begin with – although he eventually got a Nobel prize for his work!
In 1930-36, the protein nature of enzymes was finally firmly established when the protein digesting enzymes pepsin, trypsin and chymotrypsin from the gut were extracted and crystallised.
From then until today scientists have extracted more and more enzymes and have used a variety of techniques to look at their structure to give us our current level of understanding of these vital molecules.
The development of biotechnology means enzymes are now used in everything from paper and food manufacture to genetic engineering.