This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Science (applied)
Science (applied)
Enzymes are found in all organisms. What types of enzymes are there – and how do we name them?
Intracellular enzymes


Intracellular enzymes are involved in all the reactions within an individual cell or an entire organism that bring
about the shape and form of the organism and the production of new organisms in reproduction.
Extracellular enzymes

Carnivorous plants such as this pitcher plant
use the extracellular digestive enzymes of
bacteria and fungi as well as some they
produce themselves to break down the
bodies of the animals they capture.
Most enzymes have several names:
Some enzymes are still known by old and very unsystematic names that tell you very little about them – for example pepsin and trypsin.
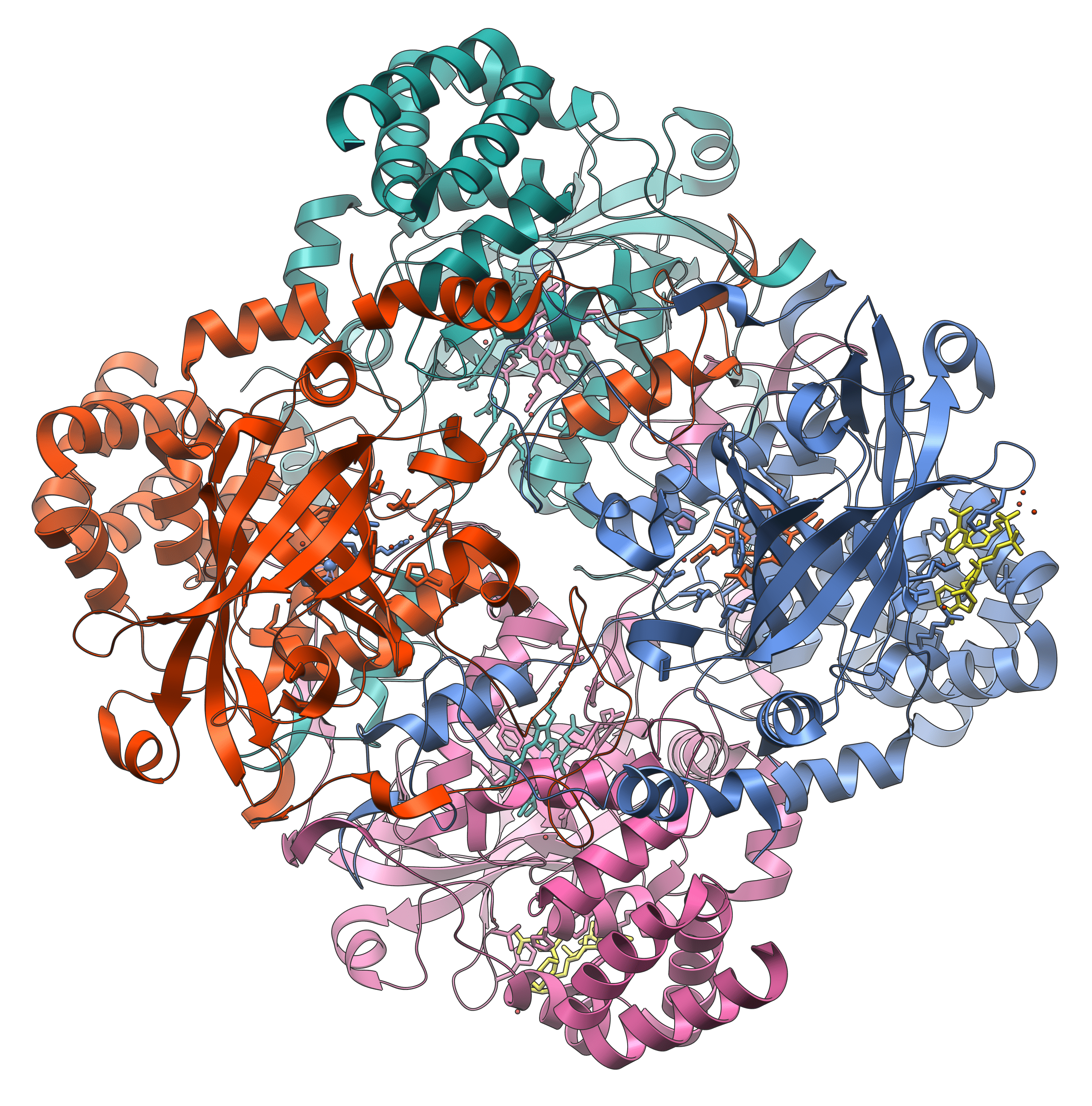
The enzyme catalase, which contains four haem prosthetic groups (Vossman, CC BY-SA 3.0).
Many enzymes need an extra, non-protein part to function properly. These non-protein parts may be ions or molecules and are referred to as cofactors. When inorganic ions act as cofactors they bind to the enzyme-substrate complex making it more efficient. Other cofactors form an integral part of the enzyme molecule and are vital for it to function at all. An example of this is salivary amylase which works more efficiently in the presence of chloride ions.
Prosthetic groups are organic molecules that are very tightly bound to an enzyme where they act as cofactors. Haem, a flat ring molecule containing iron, is the prosthetic group for a number of enzymes including cytochrome oxidase (cellular respiration) and catalase (breaks down hydrogen peroxide to water and oxygen). Haem is a permanent part of the structure of these molecules.
Coenzymes are cofactors that are complex non-protein organic molecules that are loosely associated with an enzyme. They transfer chemical groups, atoms or electrons from one enzyme to another. Many coenzymes are vitamins or vitamin derivatives e.g. nicotinamide adenine dinucleotide (NAD) is a coenzyme that plays an important role in cellular respiration and other metabolic pathways.