This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Science (applied)
Science (applied)
One technique used by scientists to investigate the role of enzymes in a cell is to inhibit an enzyme and see what does, or doesn’t, happen. Scientists have found that enzyme inhibition plays an important role within the cell in controlling the rate of the different reactions.
There are a number of different types of enzyme inhibition, and each tells us something about the structure and function of these biological catalysts.
In reversible inhibition an enzyme is not permanently inhibited or damaged. The inhibition can be reversed when the inhibitor is removed. There are two different types of reversible inhibition:
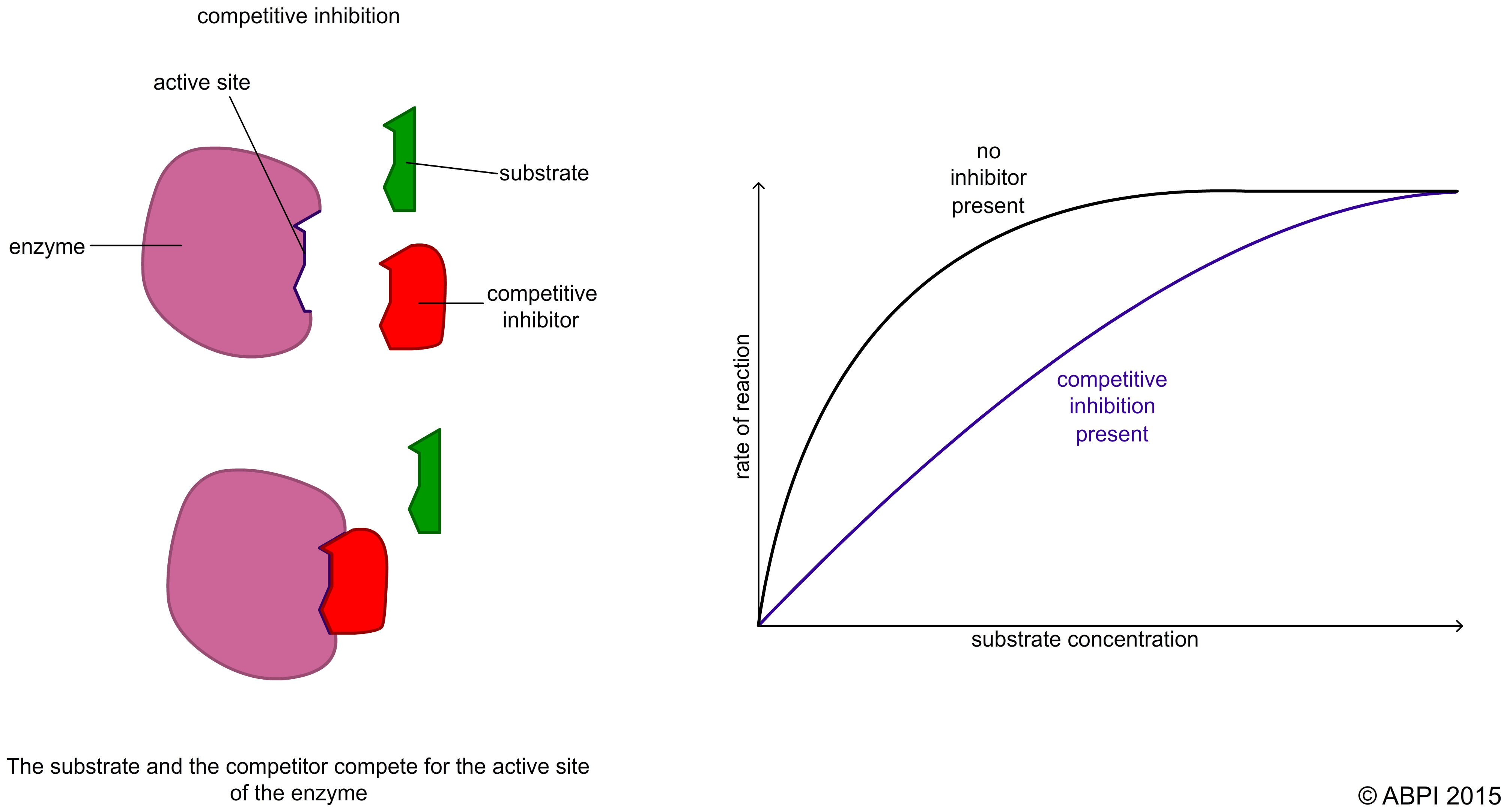
In competitive inhibition the inhibitor is very similar in shape to the normal substrate. It binds to the active site to form an inhibitor-enzyme complex. This reduces the number of enzyme molecules available for the substrate molecules to bind to. As a result less catalysis takes place and so the rate of the reaction slows down.
Because the two molecules are competing for the same active site, the rate of the reaction depends on the relative concentration of substrate and inhibitor e.g. if the amount of inhibitor is fixed, increasing the substrate concentration increases the rate of the reaction.
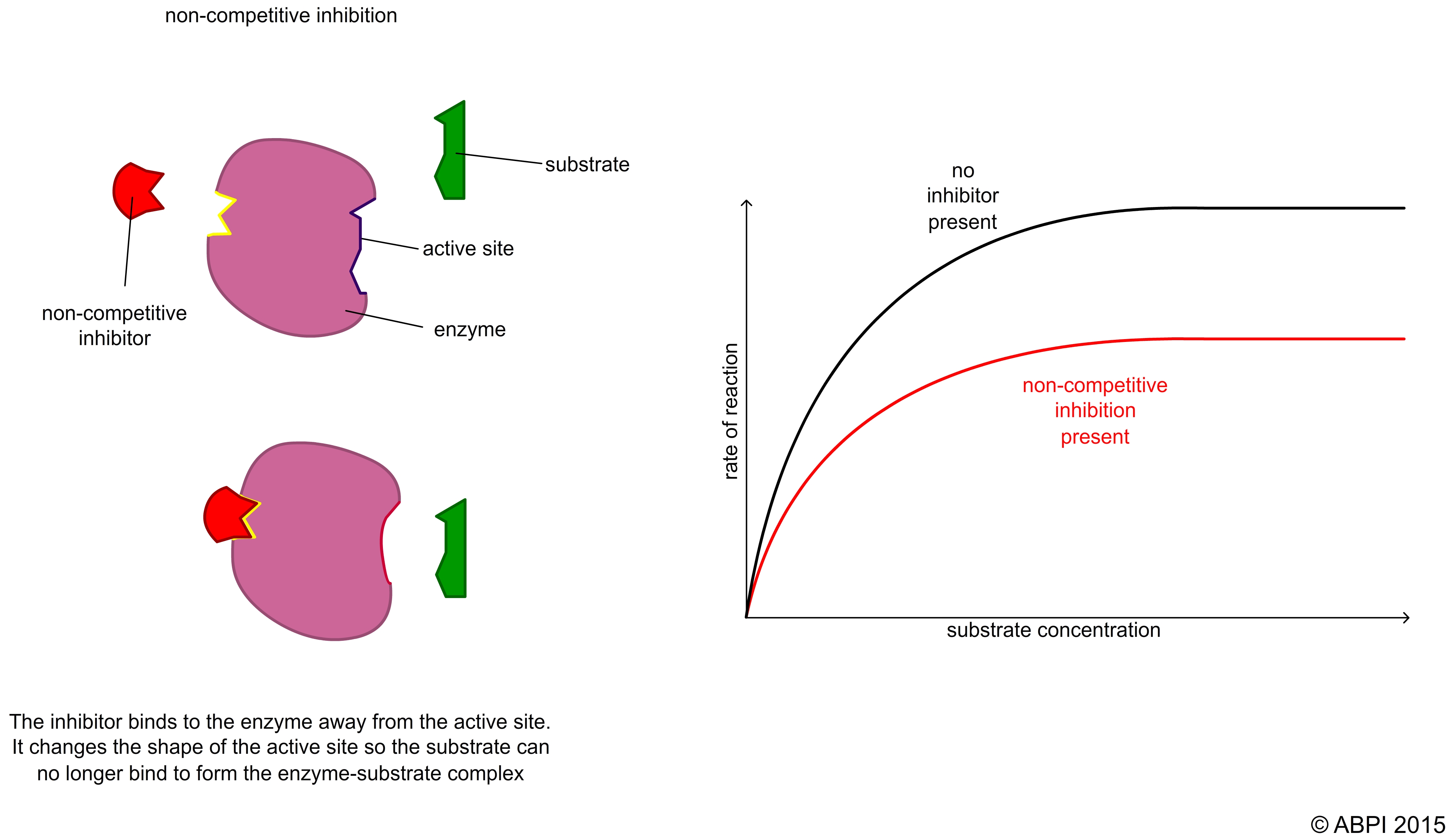
In non-competitive reversible inhibition, the inhibitor does not compete with the substrate for the active site. It binds to a different region of the enzyme. This is sometimes called allosteric inhibition (allosteric means ‘another place’ because the inhibitor binds to a different place on the enzyme than the active site). The result of the binding of the inhibitor is to change the shape of the active site so the substrate no longer fits into it. Because the substrate and the inhibitor are not competing for the same site on the enzyme, the concentration of the substrate makes no difference to the level of inhibition.
This type of inhibition is widely seen as a control mechanism in biochemical pathways such as glycolysis and the Krebs cycle.
In biochemical pathways, certain enzymes act to control the rate of the whole process. These are usually enzymes affected by non-competitive inhibition by an end product of the process. This exerts a level of fine control to ensure that metabolic pathways are tuned to the needs to the cell or organism.
1) The enzyme phosphofructokinase catalyses one of the early reactions in glycolysis:
fructose-6-phosphate → fructose-1,6-diphosphate
Phosphofructokinase is activated by high levels of its substrate fructose-6-phosphate and by ADP. It is inhibited by high levels of ATP and citrate (a compound from the Krebs cycle. When there is plenty of ATP or the products of the Krebs cycle build up, glycolysis slows down. Conversely, when the cell needs ATP for metabolic reactions and/or the components of the Krebs cycle are in short supply, glycolysis is speeded up to supply the needs.
2) In the Krebs cycle, the enzyme succinate dehydrogenase removes two hydrogen ions from succinate to form fumarate – both intermediates of the Krebs cycle. Fumarate acts as a competitive inhibitor of the enzyme, so that if the products start to build up the whole process is slowed down. But if levels of succinate build up, the inhibition is overridden and the reaction speeds up. This gives a very precise control over the rate of Krebs cycle.
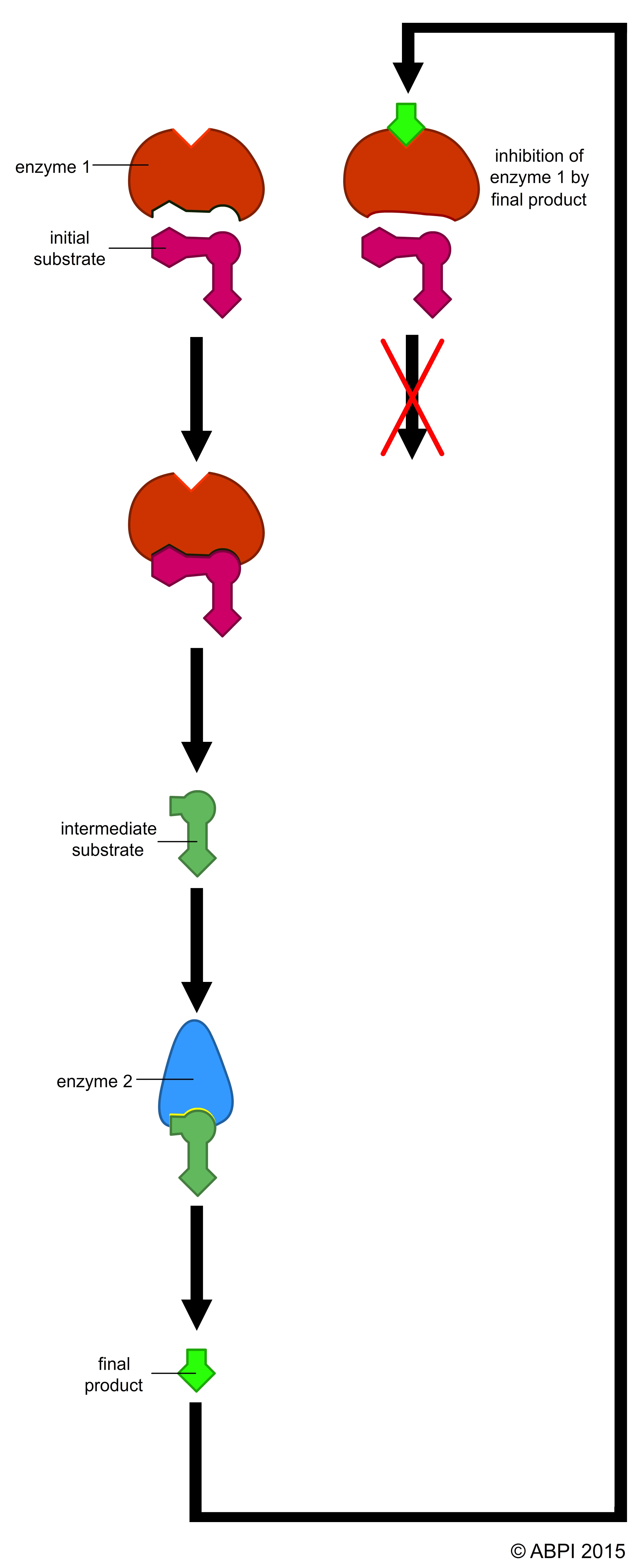
Competitive and non-competitive enzyme inhibition and end-product inhibition
In irreversible inhibition the inhibitor forms a permanent covalent bond to the enzyme, either to the active site or elsewhere. It changes the shape and structure of the molecule in a way that cannot be reversed. Irreversible enzyme inhibition is never used within cells to control metabolic pathways. Poisons such as arsenic, cyanide, mercury and many of the nerve gases used in chemical warfare often have their effect as enzyme inhibitors. For example some poisons combine with and inactivate acetyl cholinesterase, the enzyme that destroys the neurotransmitter acetylcholine in the synapse after a nerve impulse has been transmitted from a motor neurone to a muscle. When the enzyme is inhibited, the muscles go into prolonged spasms and death results as breathing and swallowing become impossible.