This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Science (applied)
Science (applied)
In a chemical reaction, chemical bonds are broken and reformed. The breaking of chemical bonds requires energy and the formation of bonds releases energy. If more energy is released as bonds form than is taken in to break the bonds, the reaction is exothermic. If more energy is taken in than is released, the reaction is endothermic. All reactions, whether exothermic or endothermic, require energy to get started. This is known as the activation energy.
One way of supplying the activation energy for a chemical reaction is to heat the reactants. Raising the temperature gives the individual particles more energy so they are more likely to collide with enough energy to react. However many reactions need very high temperatures to overcome the activation energy. This wouldn’t work in living organisms as the molecules that make up the cells and in particular the proteins would denature and the cell would die.
Enzymes are biological catalysts.
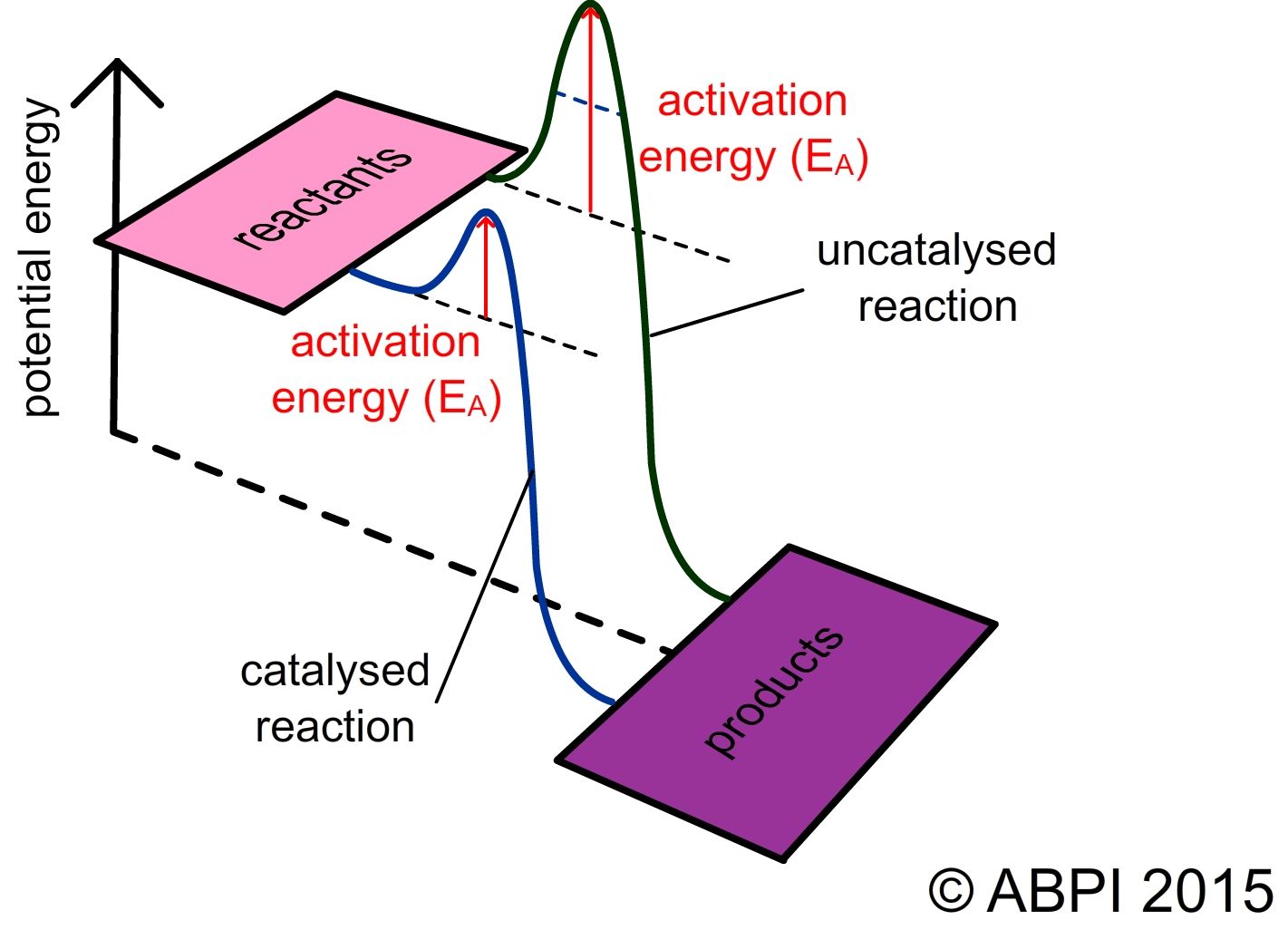
This diagram shows the energy profile of reaction with and without a catalyst.
Organisms live in many different environments, from deserts to cloud forests, and from frozen waterfalls to temperate woodlands. They may be made of one cell or billions. Wherever they live, they need a way of making sure that the reactions inside them take place at the right speed and without interfering with other reactions. Enzymes make this possible.




Enzymes control cell reactions whatever the organism and wherever it is growing.