This topic takes on average 75 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Biology
Biology
Proteins have a primary, secondary, tertiary and sometimes a quaternary structure, and each aspect of the structure is important for the protein to carry out its functions. The bonds which hold amino acids together are peptide bonds. However, the complex three-dimensional shapes of proteins which enable them to carry out their functions in the cells and the body are created and held together by hydrogen bonds, ionic bonds and disulphide bonds.
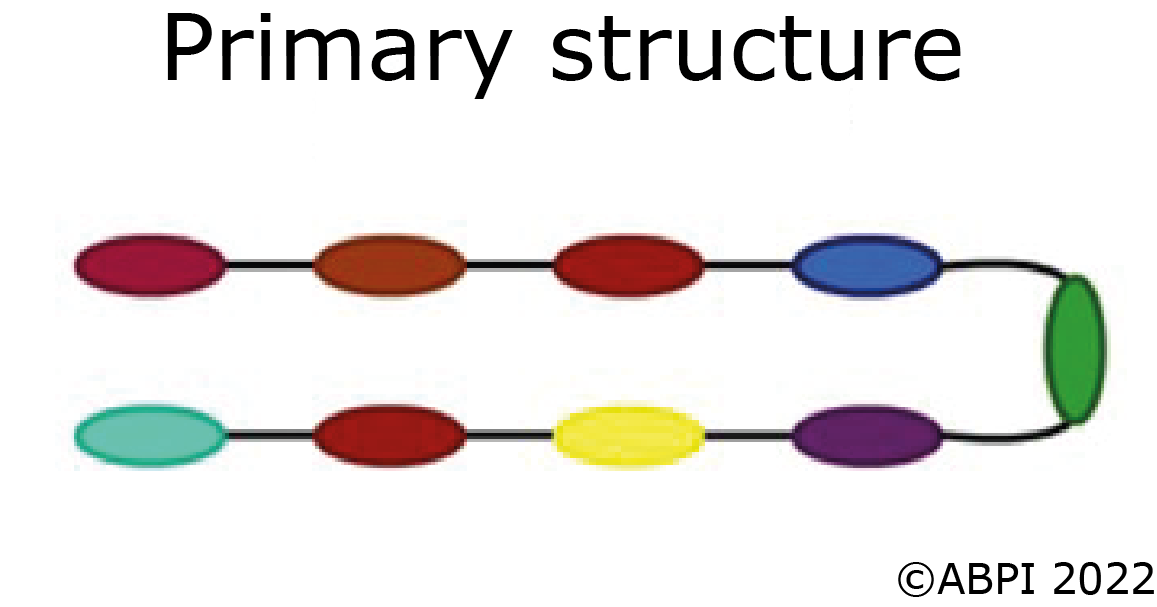
The primary structure of a protein is the order of the amino acids joined together to form the polypeptide chain.
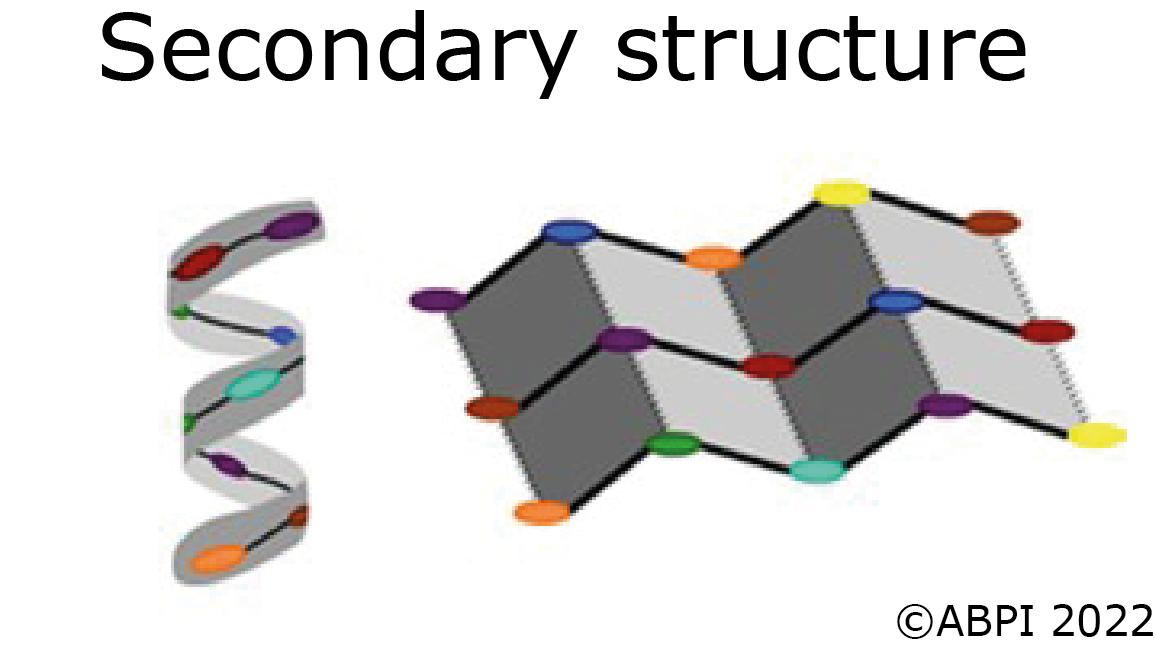
The secondary structures of proteins - α-helices and β pleated sheets – are held together by hydrogen bonds between polar molecules in the backbone of the polypeptide chain. Hydrogen bonds are relatively weak but there are many of them.
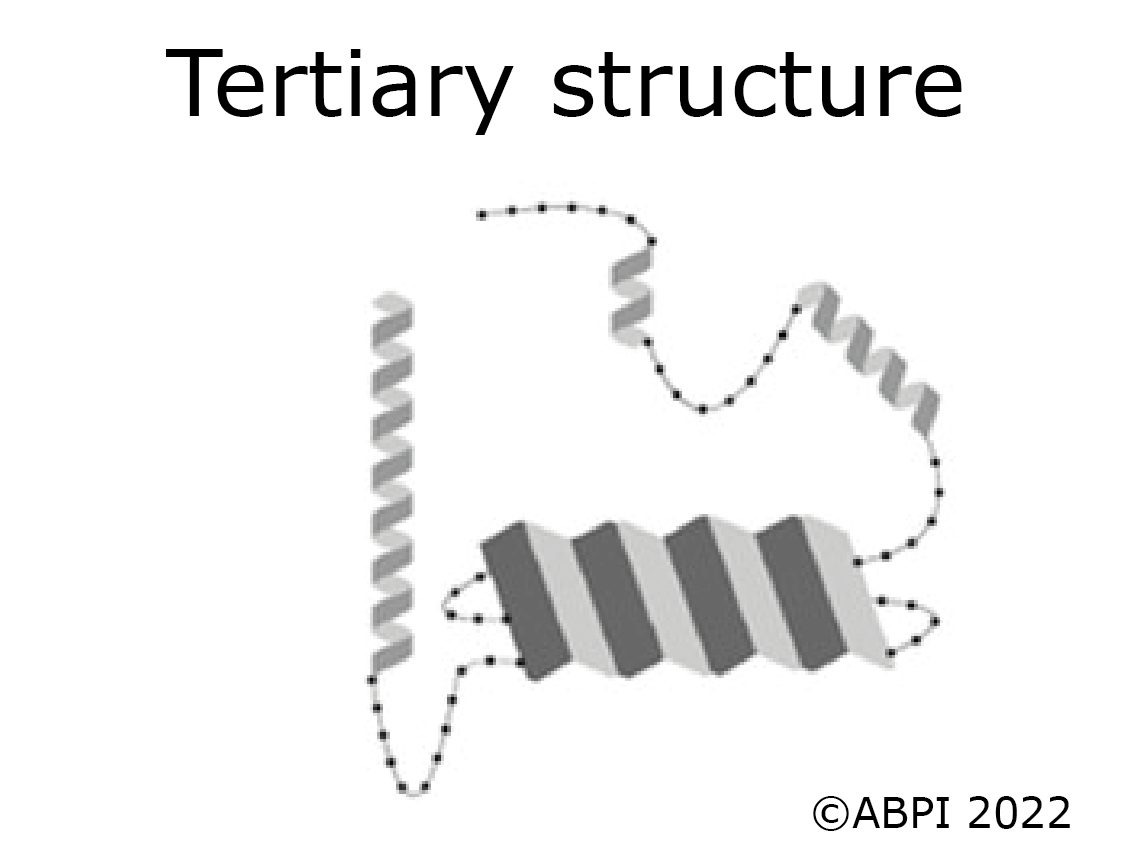
The tertiary structure of a protein is produced when the secondary structure of the polypeptide chain is folded up - for example to form a globular protein such as an enzyme. The folds are held in place by
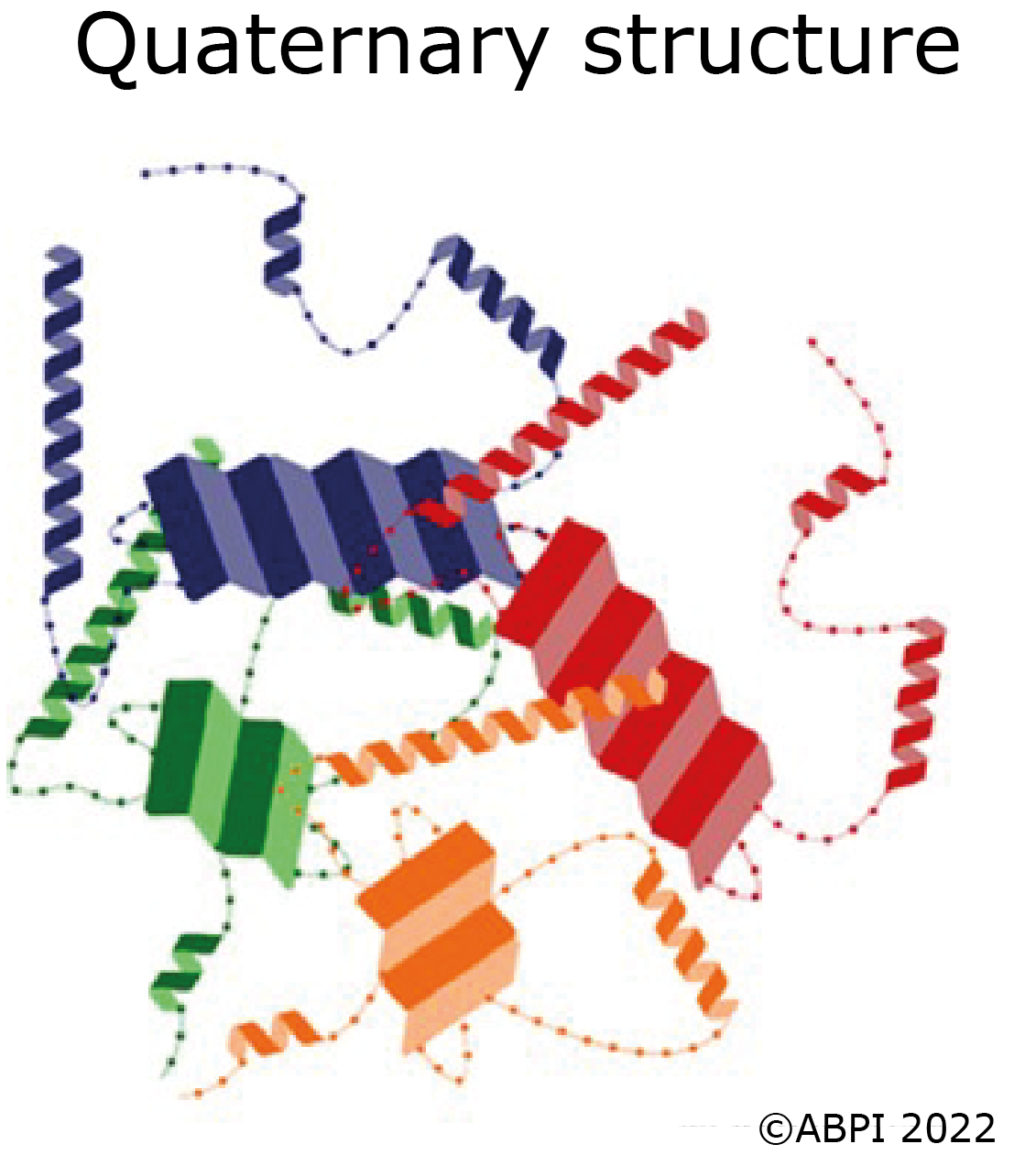
In some proteins several polypeptide chains fit together to form a large biologically active molecule e.g., haemoglobin. The quaternary structure refers to the way the different polypeptide chains fit together and are held in place by hydrogen bonds along with some ionic bonds and disulphide bonds.
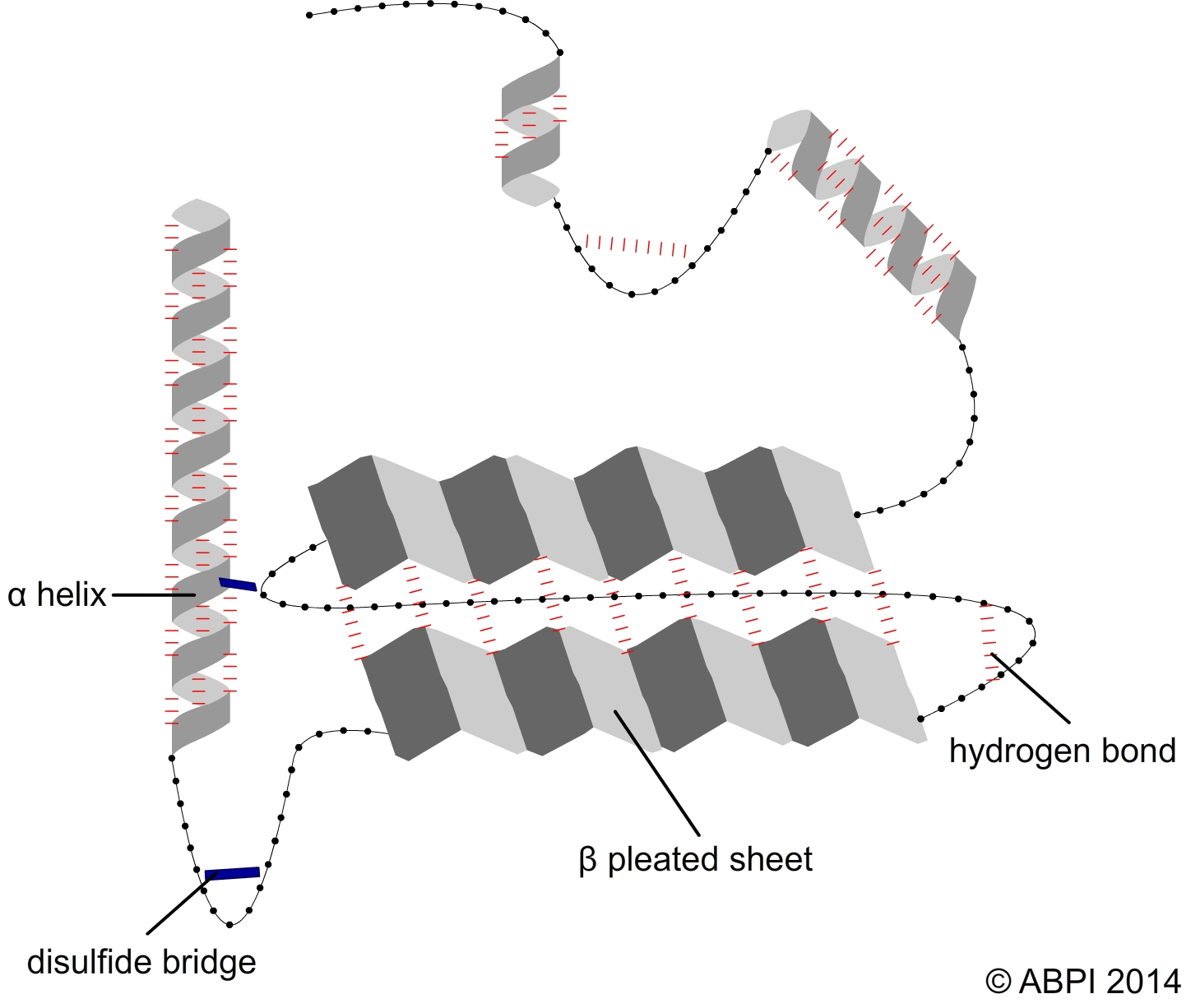
Hydrogen bonds and sulfur bridges create the complex 3-D shapes of proteins
The structure and functions of proteins are affected by both temperature and pH. Understanding the structure of proteins enables you to understand how temperature and pH have their effect.