This topic takes on average 75 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Biology
Biology
What have the cell walls of fungi, the tough outer coverings of insects, the cell walls of plants, the energy store in the human liver and the fuel for respiration in cells got in common? They are all carbohydrates, a group of organic compounds containing carbon, hydrogen and oxygen which range from very small individual molecules to very large macromolecules. They are usually classified as monosaccharides (single sugars), disaccharides (double sugars), oligosaccharides (several sugars: 3-9 units) or polysaccharides (complex carbohydrates often composed of hundreds or thousands of units which form macromolecules).

Carbohydrates are everywhere!
These simple sugars contain carbon, hydrogen and oxygen and have a general formula (CH2O)n. In theory n can be any number but it is usually between 3 and 6. The monosaccharides most commonly studied include:
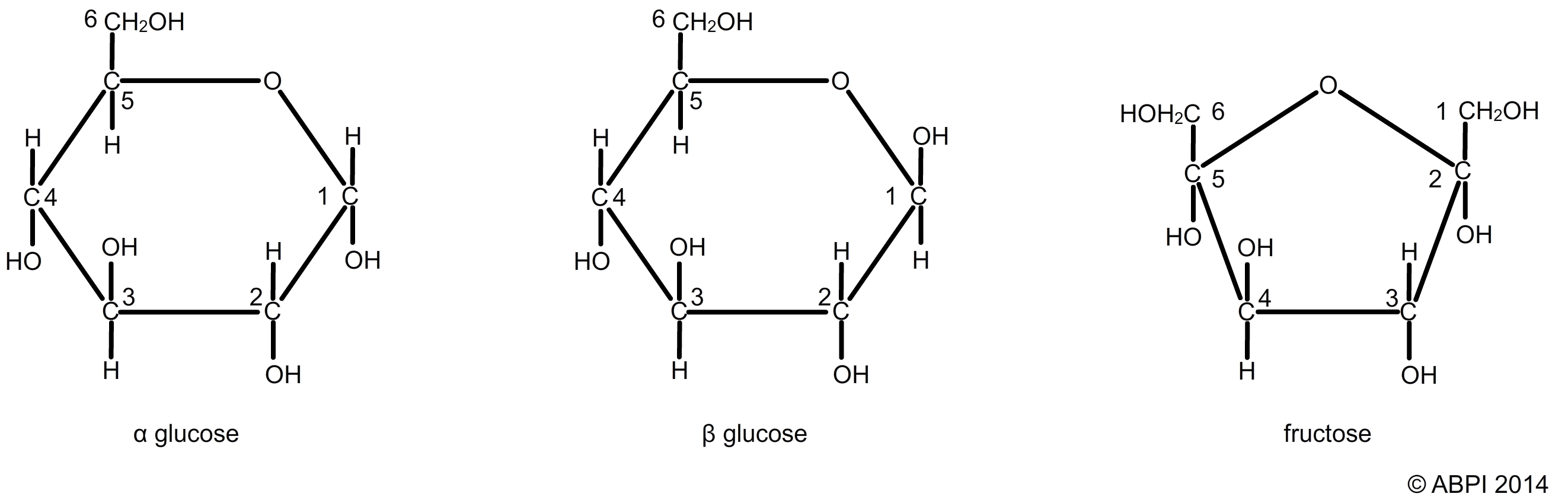
Two hexose sugars glucose (α-glucose and β-glucose) and fructose
Disaccharides are made up to two monosaccharide units joined together. Examples include the sucrose we eat (usually known as just sugar), and lactose, the sugar which sweetens milk. Disaccharides are joined using a simple condensation reaction which forms a bond between the two monosaccharide units known as a glycosidic bond. Glycosidic bonds are broken in a hydrolysis reaction to produce two monosaccharides.
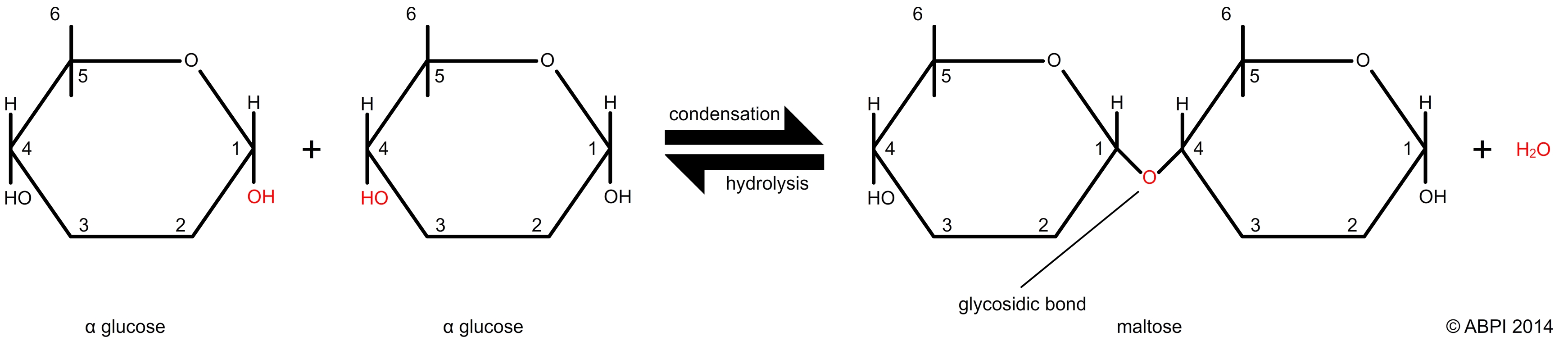
The formation and hydrolysis of a glycosidic bond
The arrangement of the atoms in monosaccharide molecules is complex due to the arrangement of the carbon bonds. As a result, several different isomers are common. This in turn affects the way bonds between the monosaccharide units are formed. Glycosidic bonds are named as a result of both the arrangement of the side groups (e.g. α and β) and by the numbers of the carbon atoms which are involved (e.g. 1,4 or 1,6). So, for example, you can have an α-1,4 glycosidic bond or a β-1,6 glycosidic bond.
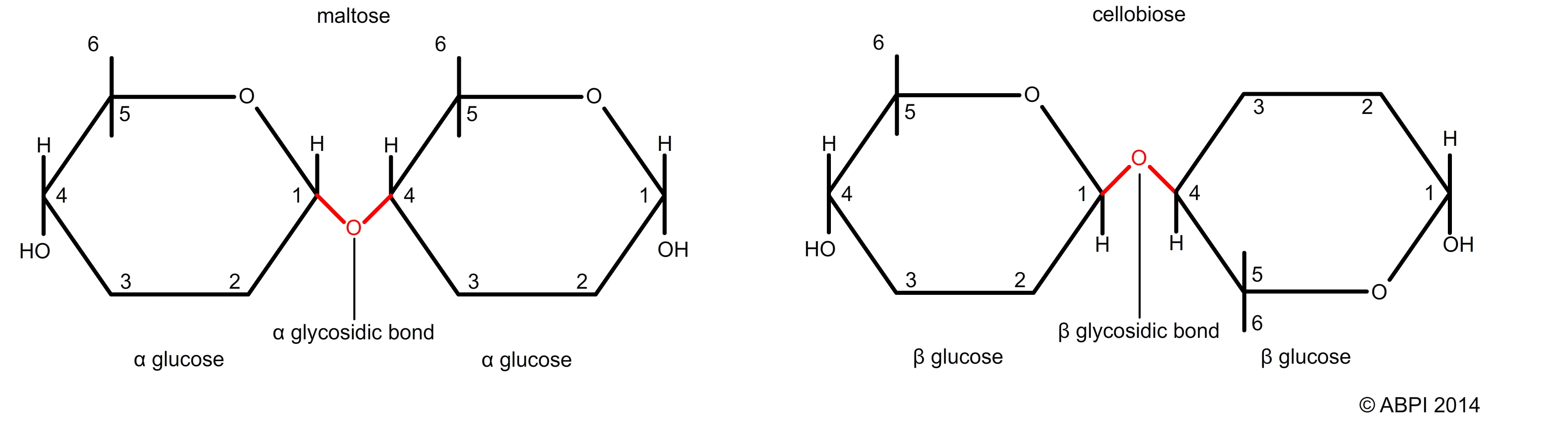
α and β glycosidic bonds
These short chains of sugar molecules are usually found associated with another molecule including proteins (glycoproteins) and lipids (glycolipids). Oligosaccharides are very important as part of the cell recognition systems on cell membranes. Oligosaccharides also seem to be important for maintaining a healthy gut flora. They are found in certain fruits and vegetables including onions, leeks and asparagus.