This topic takes on average 75 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Biology
Biology
Chemical reactions need energy to take place. The energy is needed to break the bonds in molecules to enable new bonds to form. One molecule acts as the common energy currency in all cells – adenosine triphosphate, or ATP. ATP is synthesised in the mitochondria of the cells. Anything which interferes with the synthesis or breakdown of ATP in the cell rapidly leads to the death of the cell and the whole organism.
ATP is made up of
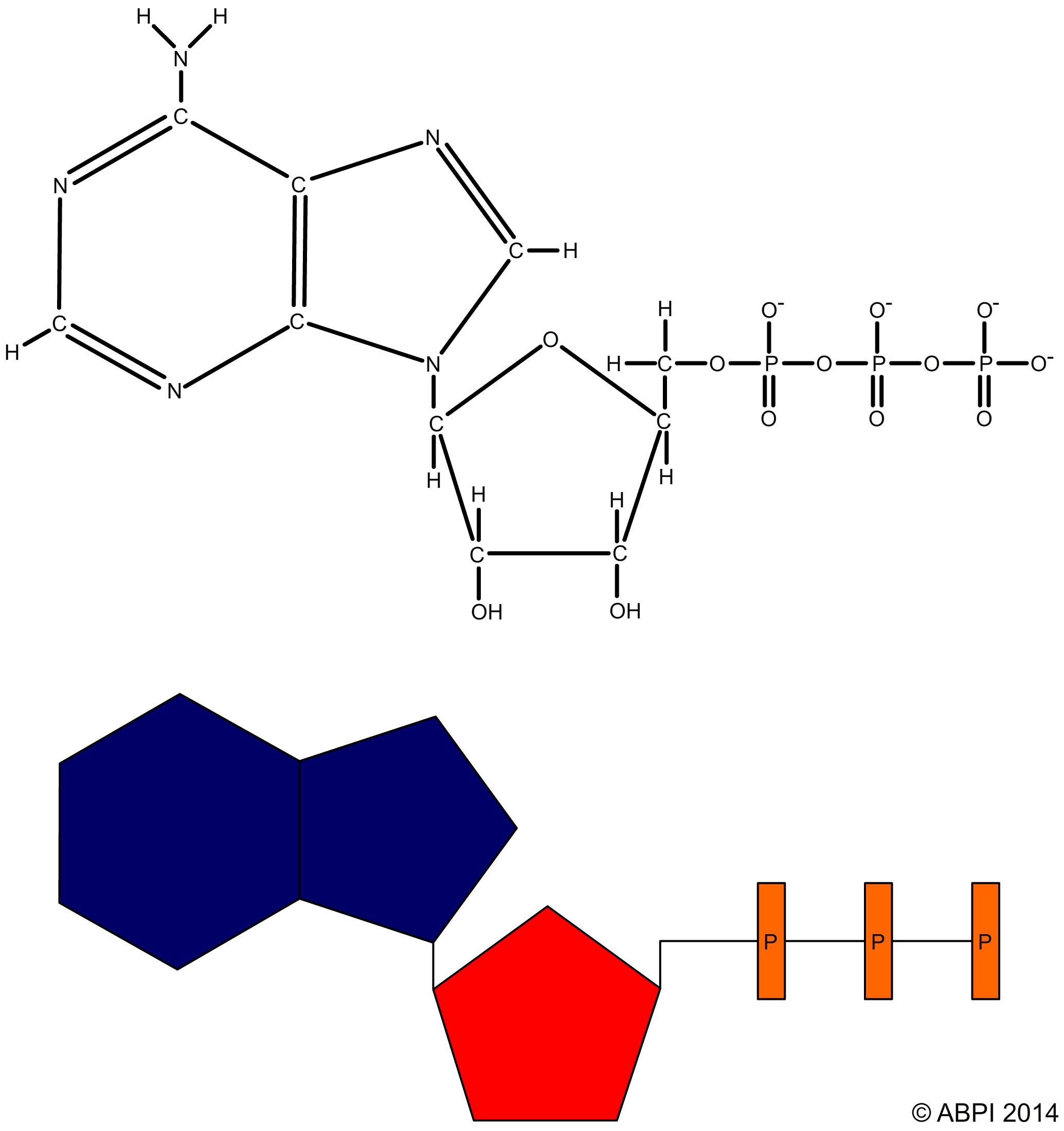
ATP acts as an energy store. When the final phosphate bond of an ATP molecule is broken by the enzyme ATPase in an exothermic hydrolysis reaction, around 30.5 kJ of energy is released per mole of ATP. Some of this energy is lost as heat but most is available to drive other reactions in the cell such as muscle contraction, protein synthesis or active transport. The products of the reaction are adenosine diphosphate, or ADP, and inorganic phosphate ions (Pi).
ATP + H2O → ADP + Pi ΔH -30.54 kJmol-1
The breakdown of ATP is a reversible reaction. The condensation reaction, catalysed by ATP synthase, produces ATP and water and requires an input of 30.54 kJ per mole of energy. It is usually linked to catabolic reactions such as cellular respiration.
ADP + Pi → ATP + H2O ΔH +30.54 kJmol-1
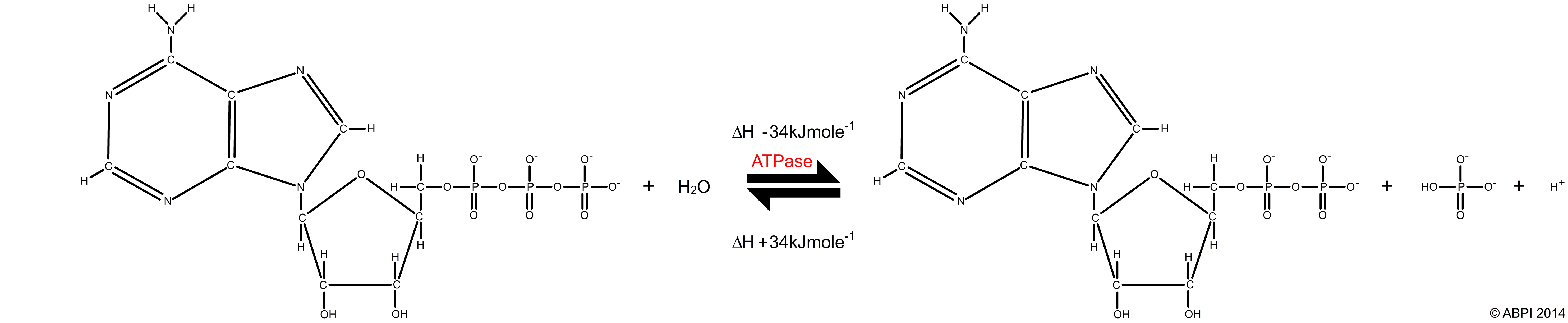
The breakdown and synthesis of ATP
The production of ATP is a very complex process. Scientists have worked out that the main way in which ATP is synthesised in the mitochondria involves the removal of hydrogen atoms from several of the intermediates in a metabolic pathway – for example during cellular respiration. Hydrogen atoms are picked up by hydrogen carriers which become reduced. Electrons from the hydrogen atoms are then passed along a series of electron carriers known as the electron transport chain. The process involves a number of reduction-oxidation reactions through a series of electron carriers known as the electron transport chain. Each reaction releases a small amount of energy which is used to drive the synthesis of a molecule of ATP.
The process is complex but very elegant – it was worked out by a scientist called Peter Mitchell and is called the chemiosmotic theory.