This topic takes on average 75 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Biology
Biology
Almost all of the material in a cell which is not water is made up of organic molecules.
Organic molecules are molecules which contain carbon and hydrogen. Many also contain oxygen and they may include a small number of other elements, including nitrogen, sulfur (sulphur) and phosphorus. Molecules containing just hydrogen and carbon atoms are known as hydrocarbons.
Each carbon atom can make four covalent bonds with other atoms. It is this property, along with the angles at which the bonds are formed, which helps to make carbon so important to life on Earth.
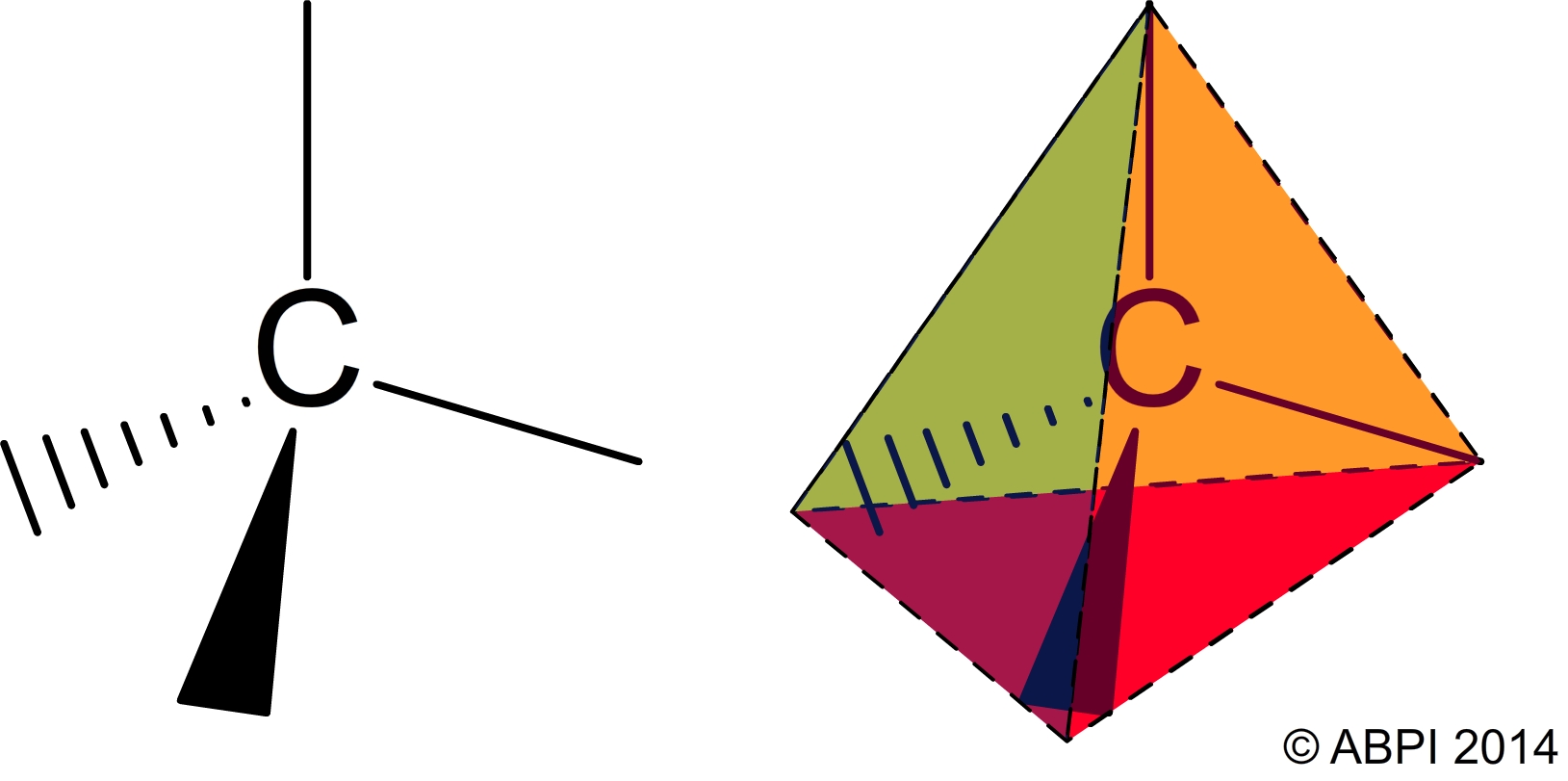
The four bonds of a carbon atom are arranged to give a tetrahedral shape.
The arrangement of the bonds of the carbon atom affects the shape of the molecules it makes when it reacts with other elements.
For example, methane (CH4) is a greenhouse gas produced by many organisms including the bacteria in the guts of ruminants such as cows, and bacteria which grow in rice paddies. It contains one carbon atom and four hydrogen atoms arranged in a typical tetrahedron.
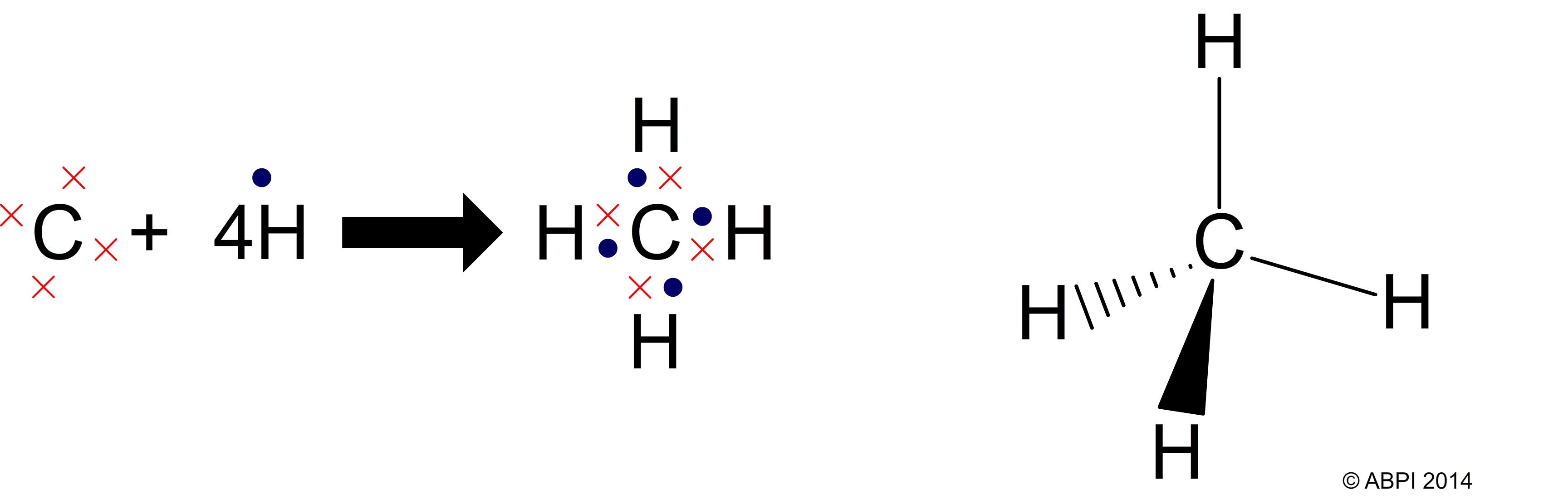
Carbon atoms can form single, double and even triple bonds, and this is another important feature of organic molecules.
The three dimensional arrangement of the carbon bonds means that organic molecules often show isomerism. Structural isomers have exactly the same numbers and types of atoms – they have the same molecular formula – but the arrangement of the atoms is different e.g. α-glucose and β-glucose. The molecules can be represented in a number of different ways – for example, the individual carbon atoms are often not shown (see the diagram below which gives two different representations of α-glucose and β-glucose).
Structural isomers
The three dimensional arrangement of the carbon bonds means that organic molecules often show isomerism. Structural isomers have exactly the same numbers and types of atoms – they have the same molecular formula – but the arrangement of the atoms is different e.g. α-glucose and β-glucose. The molecules can be represented in a number of different ways – for example, the individual carbon atoms are often not shown (see the diagram below which gives two different representations of α-glucose and β-glucose).
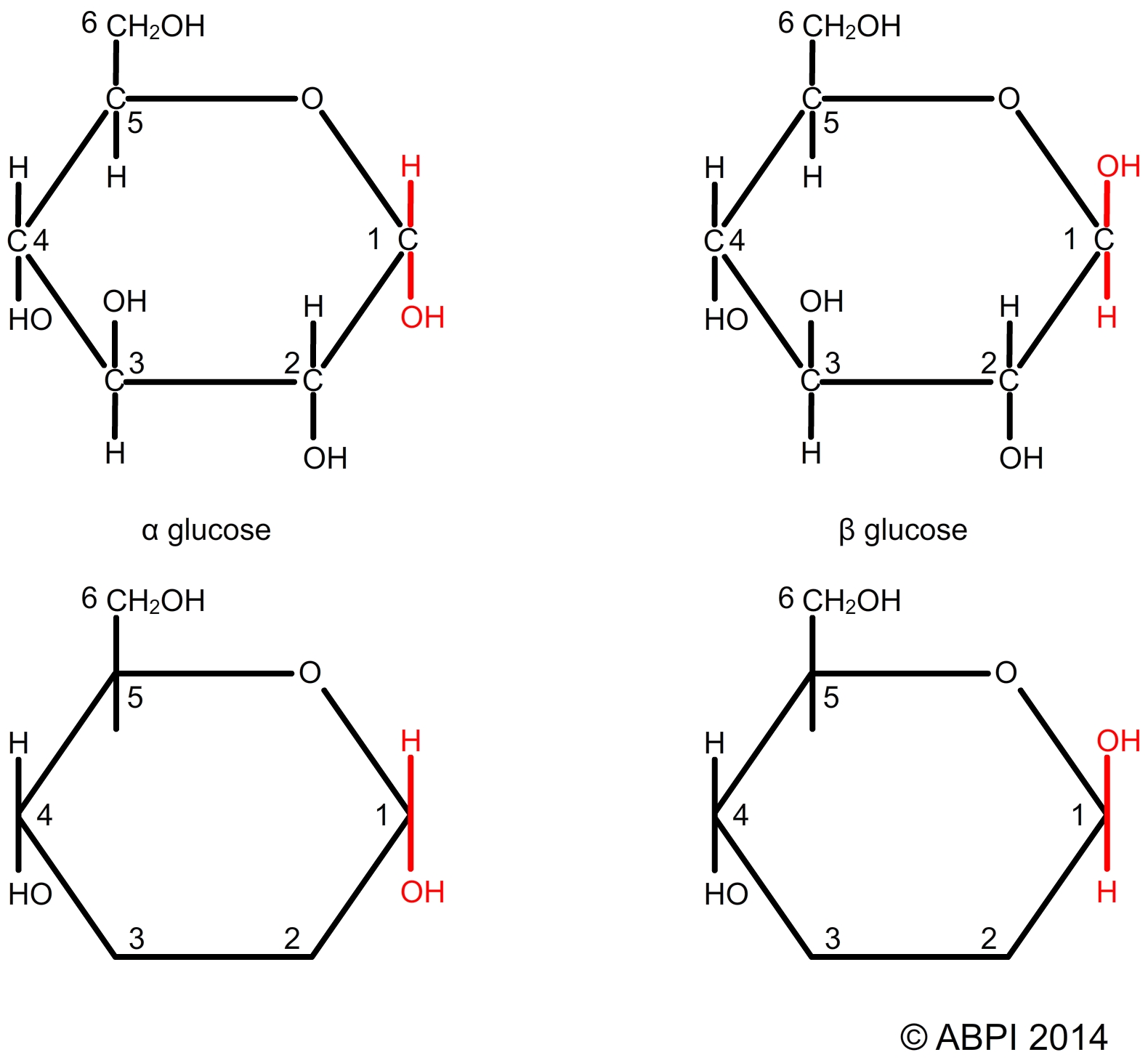
α- and β-glucose
Stereoisomers
Some organic molecules also show stereoisomerism. They are mirror images of each other and cannot be superimposed.
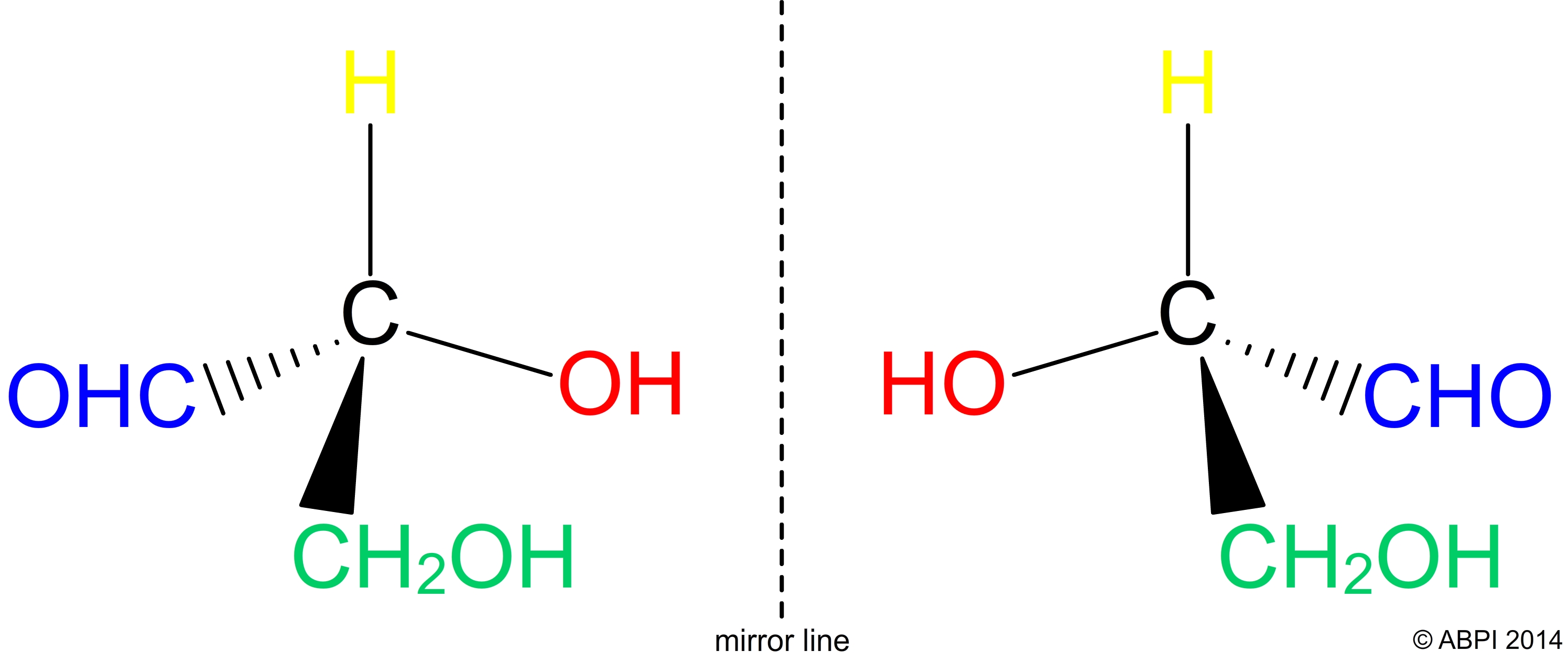
D- and L- glyceraldehyde are stereoisomers
Isomerism is important because biological systems are sensitive to it. For example, it is usually the D forms of sugars and the L forms of amino acids which are found in living organisms, because those are the forms which the enzymes in cells can recognise.
Carbon atoms bond very strongly to other carbon atoms. As a result carbon atoms tend to bond together to form very large molecules known as macromolecules. Carbon atoms can join to form long chain molecules, with other atoms and molecules attached to the chain. They can form branched chains, rings and complex three-dimensional shapes. Often a relatively small molecule called a monomer will be joined to lots of similar units to form a very large molecule called a polymer. Polymers are the most common form of macromolecule.
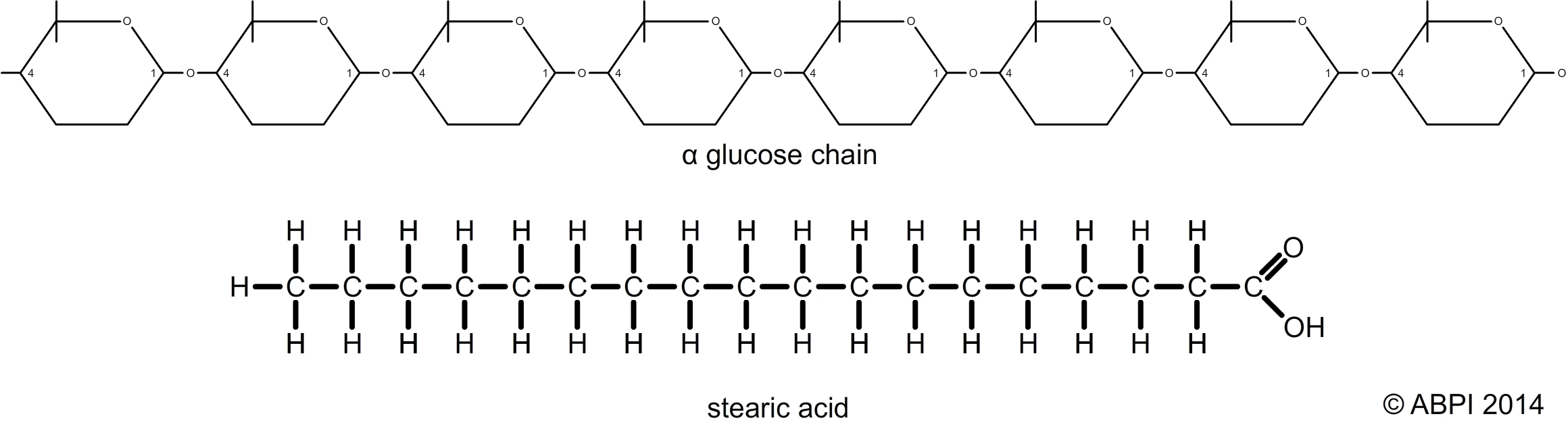
Two common macromolecules – can you work out the monomer in each?
Carbon based monomers often join together in a condensation reaction. This is a reaction in which a molecule of water is removed as a bond is formed.
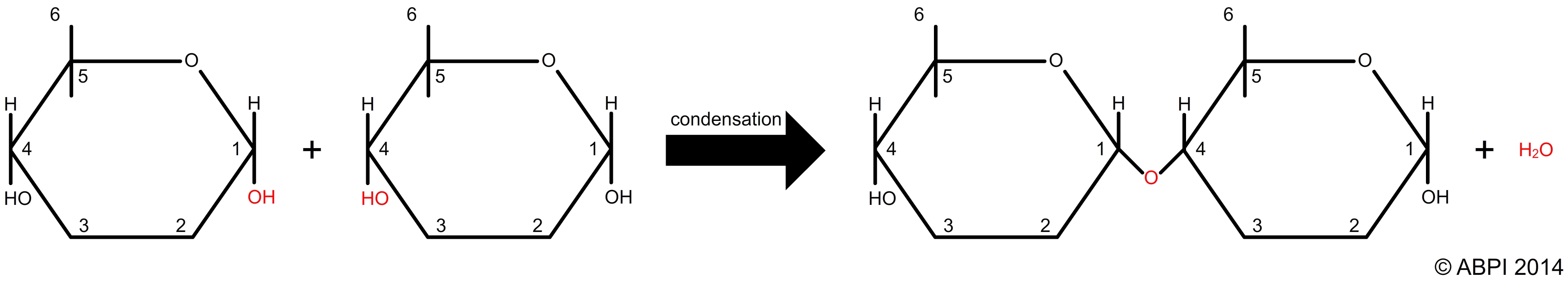 A condensation reaction
A condensation reaction
Polymers formed in this way can be broken down into their constituent monomers in hydrolysis reactions. A bond is broken by the addition of a molecule of water (hydro = water, lysis = breaking or splitting).
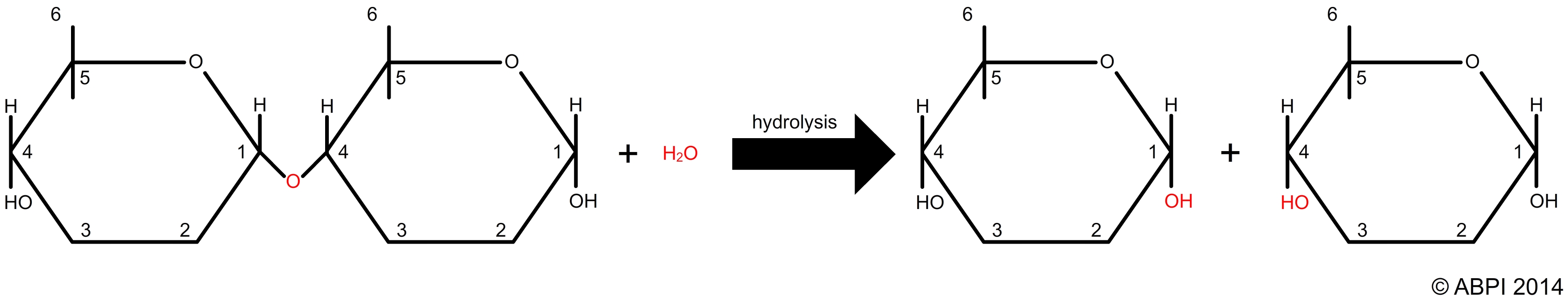 A hydrolysis reaction
A hydrolysis reaction
Many of the reactions synthesising macromolecules are reversible. The rate of the reactions in cells is enzyme-controlled. The direction of the reaction will depend on the conditions and the enzymes which are present:
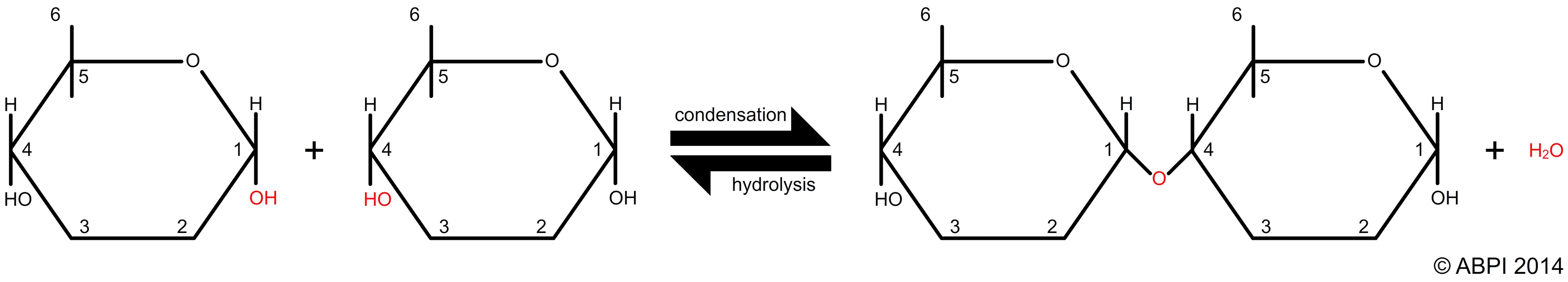 Reversible condensation-hydrolysis reaction
Reversible condensation-hydrolysis reaction