This topic takes on average 75 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Biology
Biology
Most macromolecules are polymers. Lipids are macromolecules – they can be very large indeed – but they are not polymers. Lipids are very varied and they play a number of key roles in living things that include playing an important part in the structure of cell membranes, as a high-energy food and as hormones, chemical messages which control the physiology of many living organisms.
All lipids contain carbon, hydrogen and oxygen, but they contain relatively few oxygen atoms. They all dissolve in organic solvents but do not dissolve in water. They are all macromolecules, but they contain quite a variety of different structures.
Chemically fats and oils have the same basic structure, but fats are solids at room temperature and oils are liquids. Fats and oils are very compact molecules which are used as energy stores in both the plant and animal kingdoms. Lipid-rich plant and animal tissue make energy-rich food sources. The triglycerides are made up of glycerol with 3 fatty acids attached.
Glycerol (propane-1,2,3-triol)
All of the triglycerides are based around glycerol. The IUPAC name for this molecule is propane-1,2,3-triol but it is still always referred to in biochemistry as glycerol. It is a colourless, odourless viscous sweet liquid which is the core of all the triglycerides.


(Photo credit: stevevoght, licensed under the Creative Commons Attribution-Share Alike 2.0 Generic license.)
Fatty acids are carboxylic acids and over 70 different types have been extracted from living tissues alone. They have a very long pleated backbone of carbon atoms with hydrogen atoms attached, and a carboxyl group (-COOH) at one end. The long carbon chain is often represented by the letter R in diagrams. Fatty acids vary in two main ways:
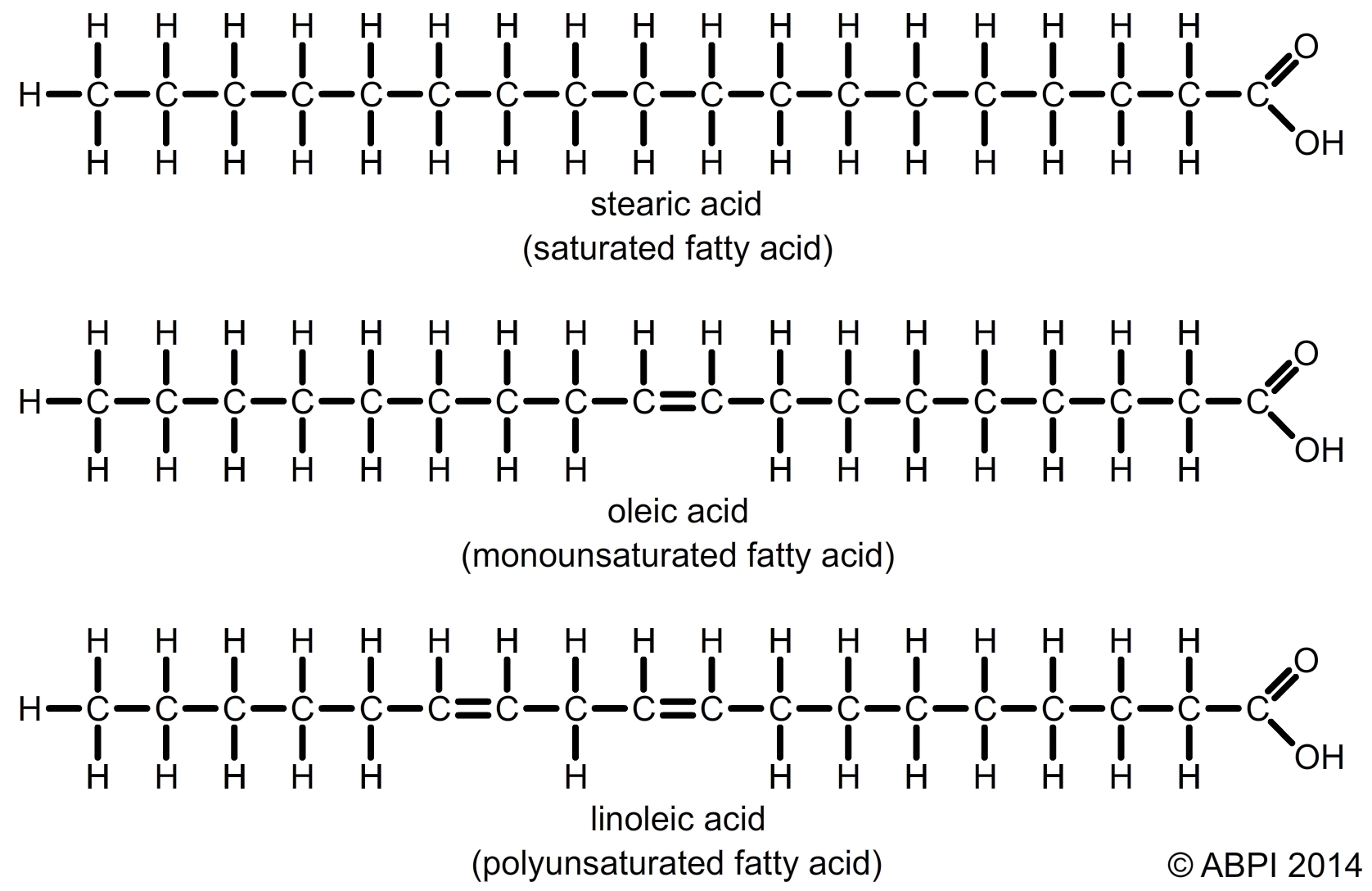
Saturated and unsaturated fatty acids
Triglycerides are formed by condensation reactions between glycerol and three fatty acids to form an ester bond. Each condensation reaction between glycerol and a fatty acid is an example of esterification. This bond in an ester can be broken down by a hydrolysis reaction.
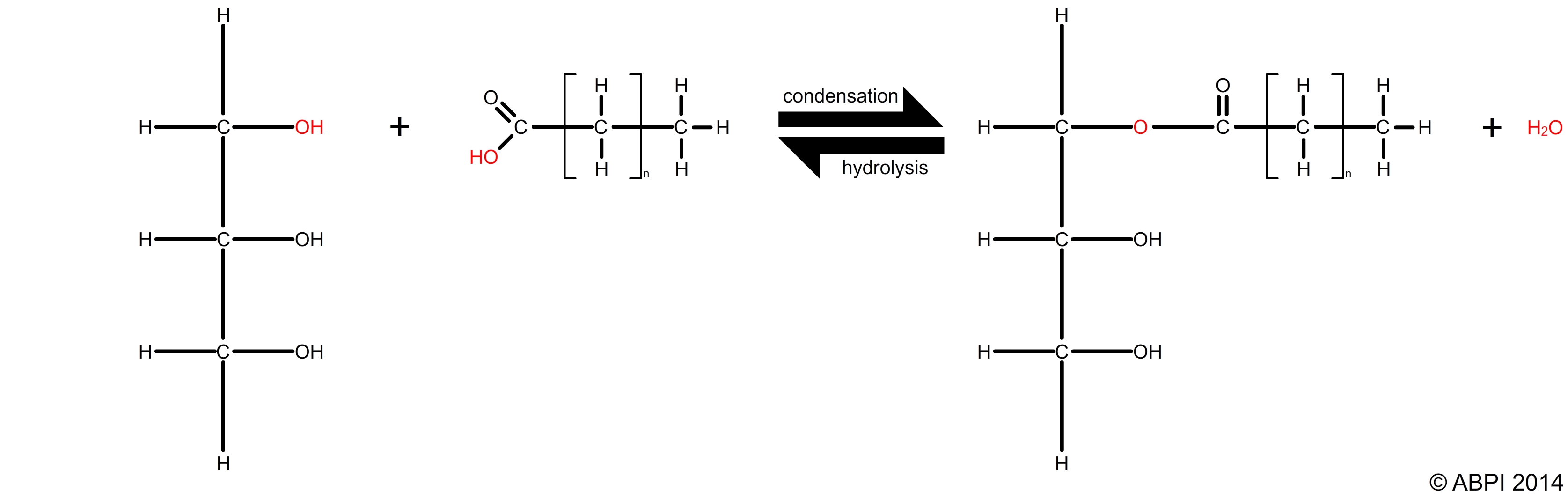
Reversible esterification and hydrolysis reactions between a fatty acid and glycerol
To better understand how glycerides are formed, watch the animation below.