This topic takes on average 75 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Biology
Biology
The properties of the chemicals which make up all organisms depend on the nature of the chemical bonds which hold them together. Remember that in chemical reactions the electrons are rearranged to ensure that the atoms within the new compound all have a stable outer shell of electrons, giving them the configuration of a noble gas. In summary:
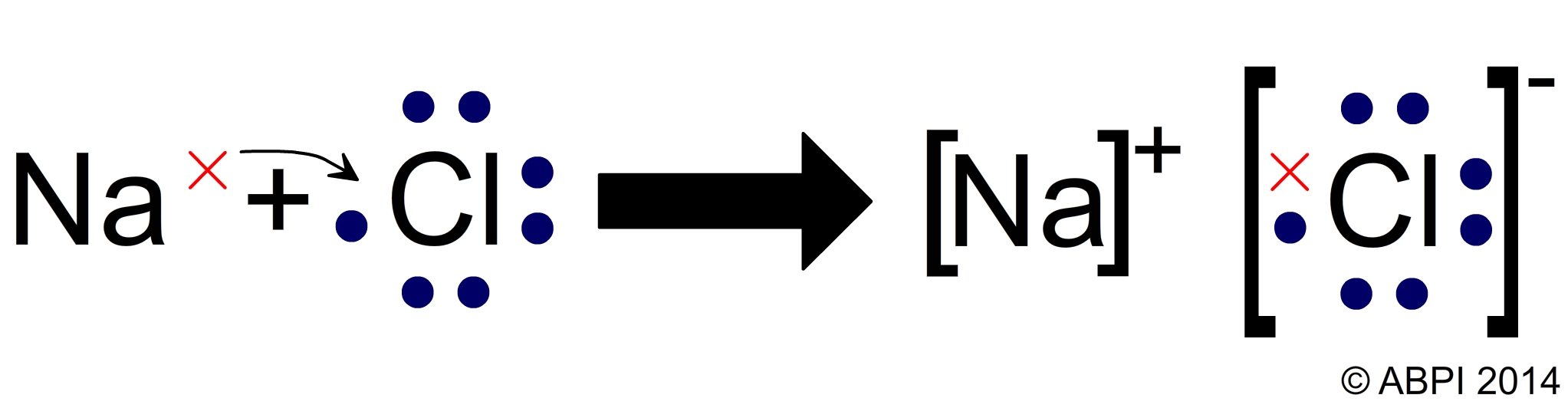
Ionic bonding between sodium and chlorine atoms
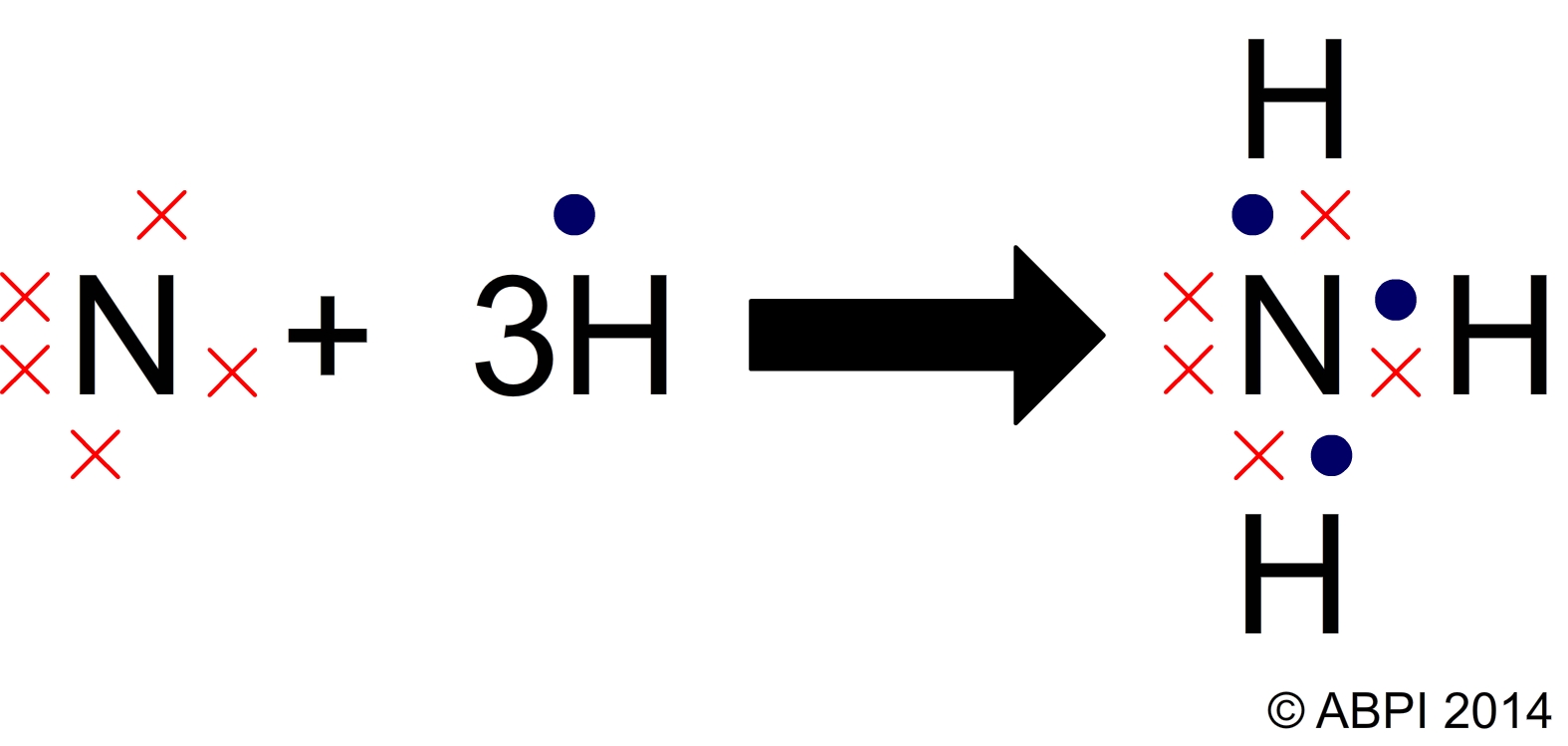
Covalent bonding between nitrogen and hydrogen atoms
Many biologically important molecules are covalent, but the negative electrons in covalent molecules are not always evenly distributed. The electrons may spend more time close to one of the atoms than to another, because some atoms are more electronegative than others. As a result, some parts of the molecule will be slightly negative and some slightly positive. The slightly charged regions are called dipoles and the charges are written as δ+ or δ-. When covalent molecules have dipoles they are known as polar molecules.
Polar molecules are held together by electrostatic forces. These are much weaker than ionic bonds, but because they form between all of the molecules in a substance, there are a lot of them and they can have a major effect on the chemistry of the compound.
Hydrogen bonds are the best known example of these dipole:dipole attractive forces. They occur when hydrogen atoms are covalently bonded to extremely electronegative atoms such as oxygen, nitrogen or fluorine. For example, when oxygen and hydrogen are covalently bonded to form a hydroxyl group (-OH), the oxygen always has the greater share of the electrons than the hydrogen and so the molecules have some polar nature.
Organic compounds often contain hydroxyl groups, giving them a polar nature. Hydrogen bonds have a huge influence of the properties of many biologically important molecules including water.