This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Human biology
Human biology
 Biology
Biology
 PSHE / Citizenship studies
PSHE / Citizenship studies
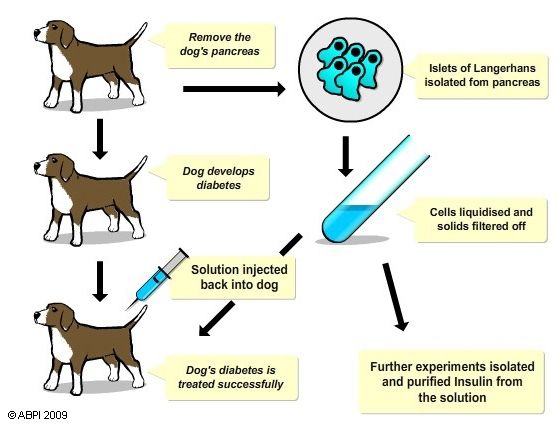
In 1921 two researchers, Fred Banting and Charles Best, were the first to discover insulin and use it to treat diabetes.
At the time that Banting and Best were looking for a cure for diabetes, there were no alternatives but to perform their experiments using animals.
To test their theory Banting and Best used 10 dogs. They made the dogs diabetic and then investigated treatments for the diabetes. The result of their research has saved the lives of millions of people.

Injecting insulin allows
individuals with diabetes to
control their blood sugar levels.
Some people have very strong opinions about testing medicines on animals. Today the use of animals is very tightly regulated and only allowed in circumstances where there are no other viable alternatives.
Using animals for biomedical research is highly regulated in the UK. The law contains these principles:
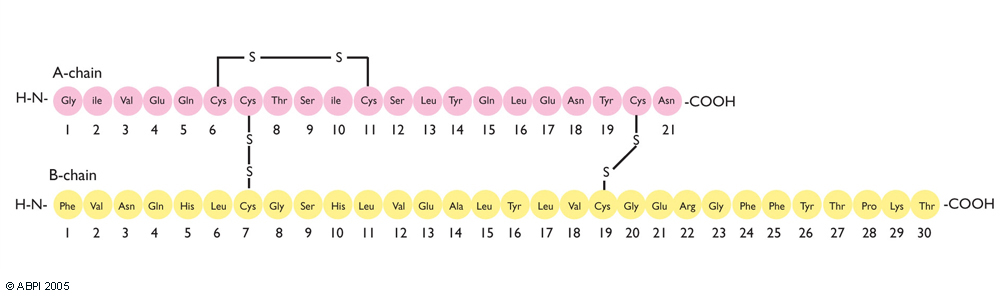
Banting and Best did not know the chemical structure of insulin. In 1955, Fred Sanger determined its amino acid sequence, and in 1969, Dorothy Hodgkin used X-ray crystallography to find its three-dimensional structure.
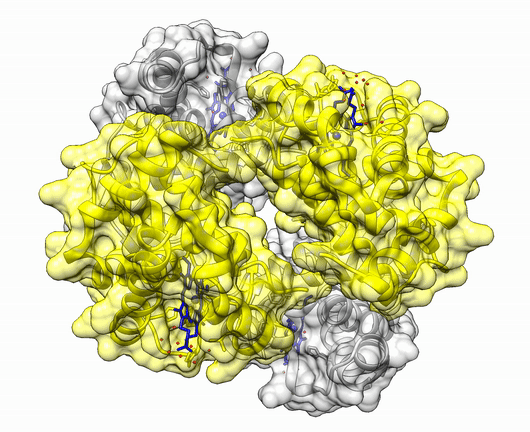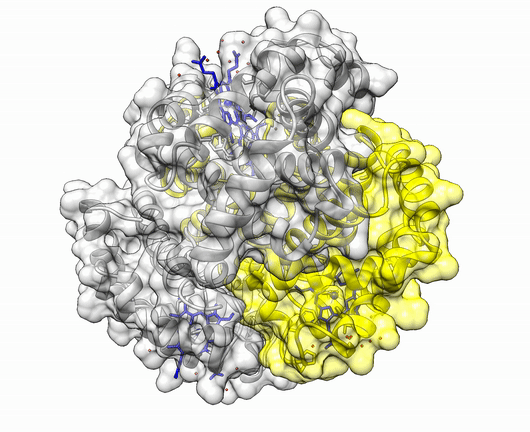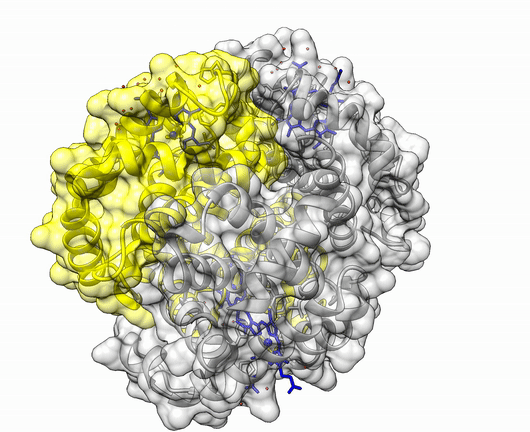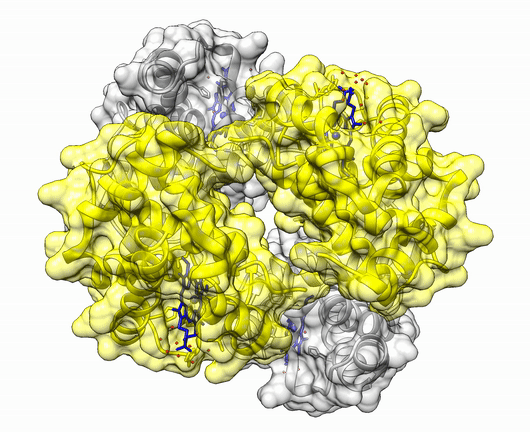
The insulin molecule acts by attaching to cell-surface receptors on its target cells. Its three-dimensional structure enables it to attach to these receptors. The shape of the insulin molecule is determined by the way the protein chains fold around each other due to hydrogen bonds and disulphide bridges.
Insulin is a protein made up of two amino acid chains.
The three dimensional structure of protein is described using four categories:
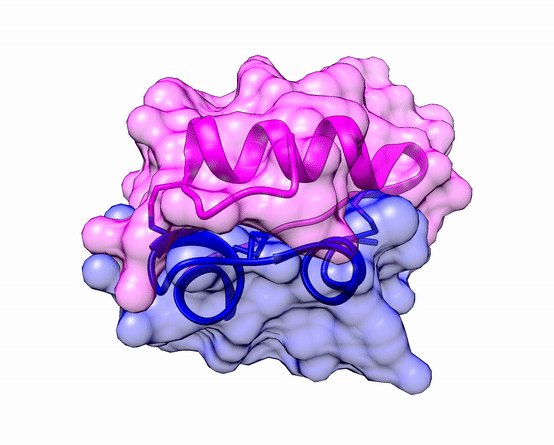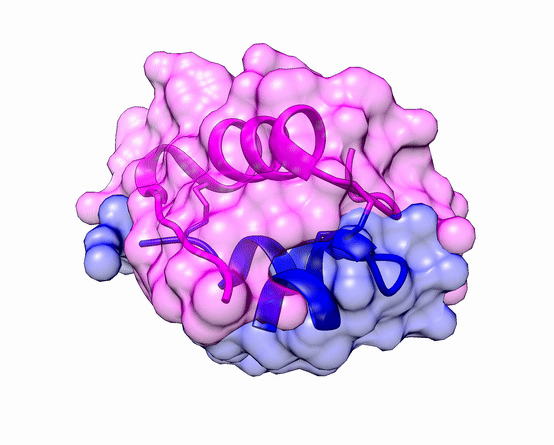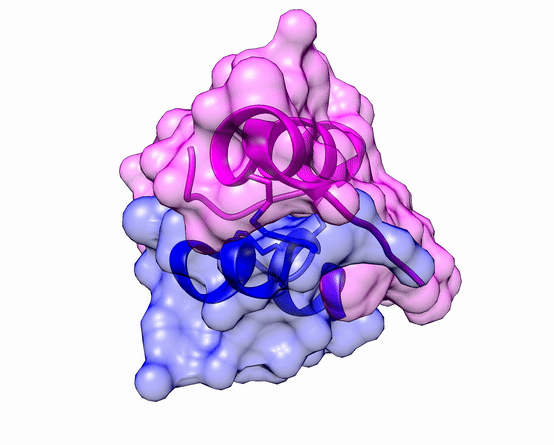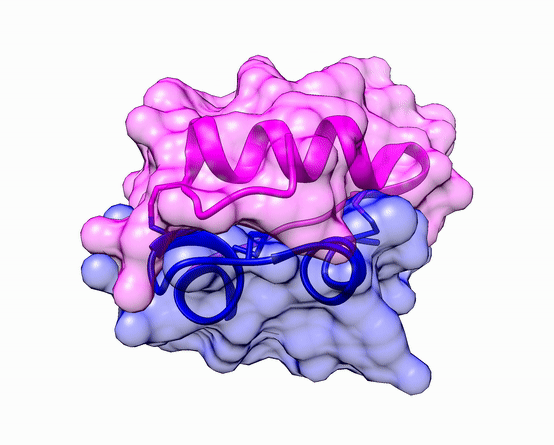
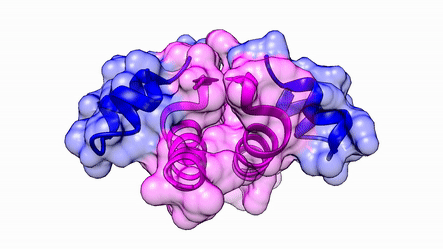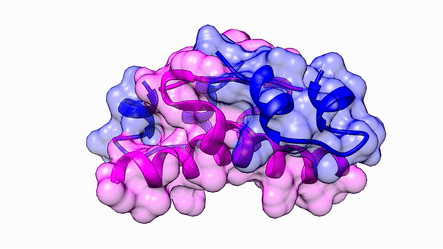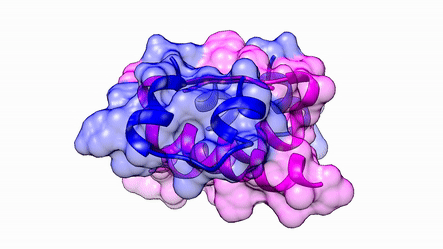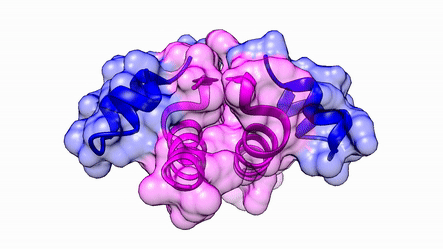
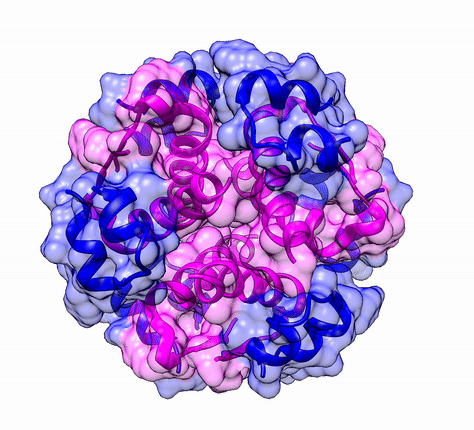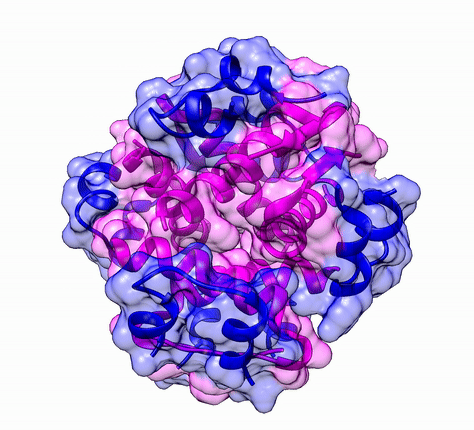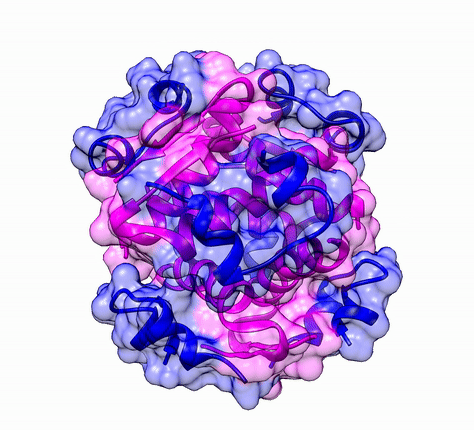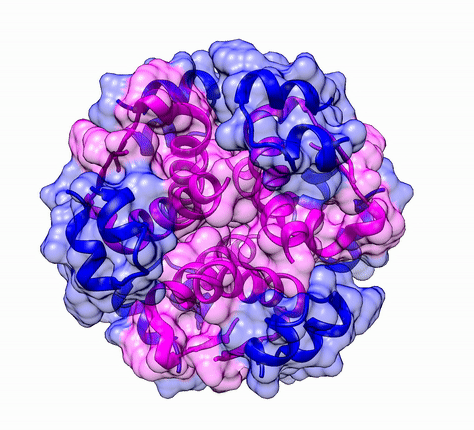
Another good example is haemoglobin. Its quaternary structure is a heterotetramer. The active conformation is formed by the arrangement of two copies of the α and two copies of the β chain. Each one binds a haem group.