This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Chemistry
Chemistry
 Science
Science
 Human biology
Human biology
In a triple bond six electrons are shared. Let’s consider ethyne, C2H2, as an example.
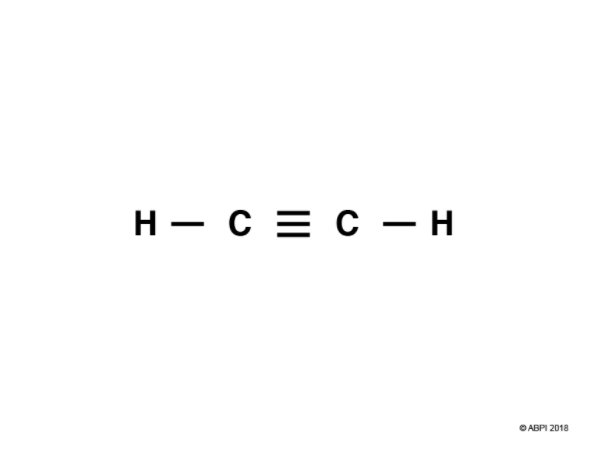
Each Carbon in ethyne has four bonds so, as before with ethane and ethene, one of Carbon’s 2s electrons is promoted to a 2p orbital.
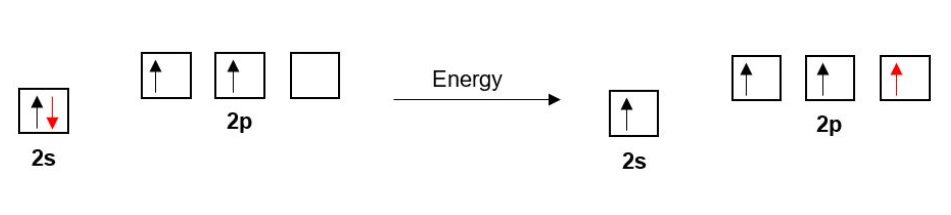
As we saw in the last section, the double bond in ethene is composed of one σ bond and one π-bond. Similarly, the triple bond in ethyne is composed of one σ-bond and two π-bonds.
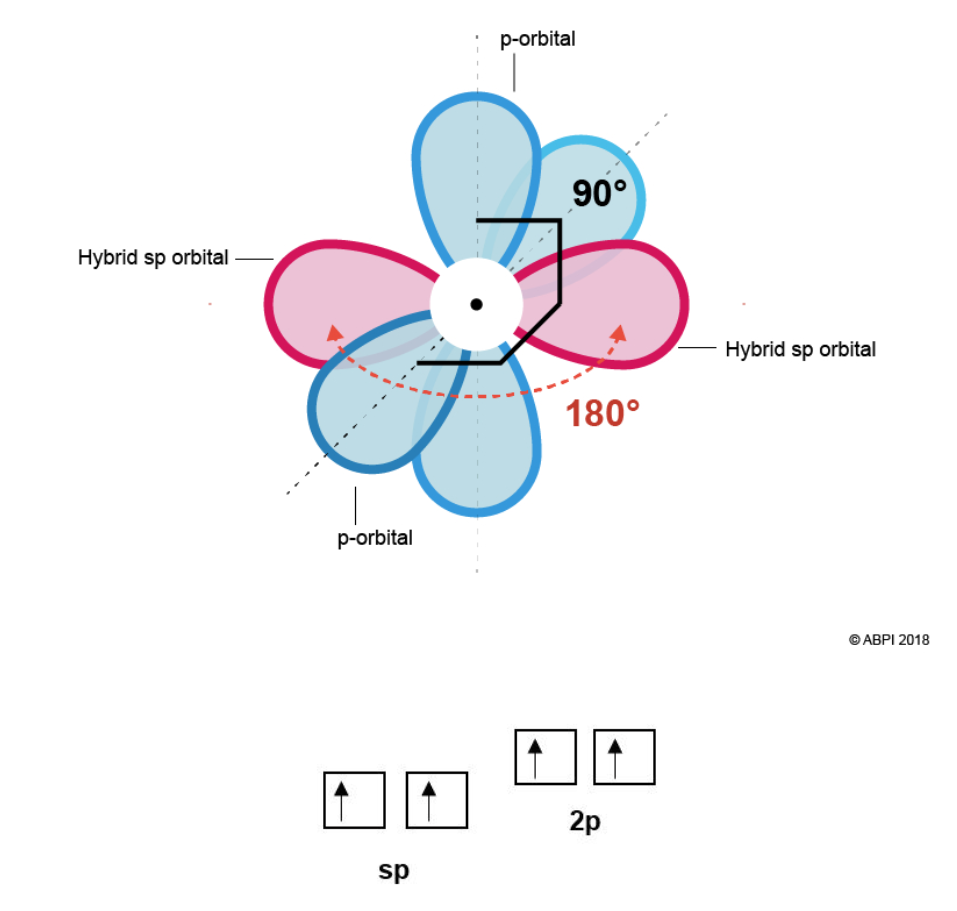
This means that each Carbon in ethyne has two σ-bonds (one to bond to the Hydrogen and one to bond to the other Carbon). To form the two σ-bonds, two hybrid sp orbitals are formed from one s orbital and one p-orbital. This leaves two p-orbitals remaining.
The two sp orbitals rearrange themselves as far from each other as possible (180° from each other). The two-remaining p-orbitals are at right angles (90°) both to the sp orbitals and to each other.
Sigma bonds form by the merging or end-to-end overlap of atomic orbitals, with resulting bonds containing a bonding pair of electrons. Two σ-bonds are formed – one between Hydrogen’s 1s orbital and Carbon’s sp hybrid orbital and the other between each of the Carbon’s sp orbitals.
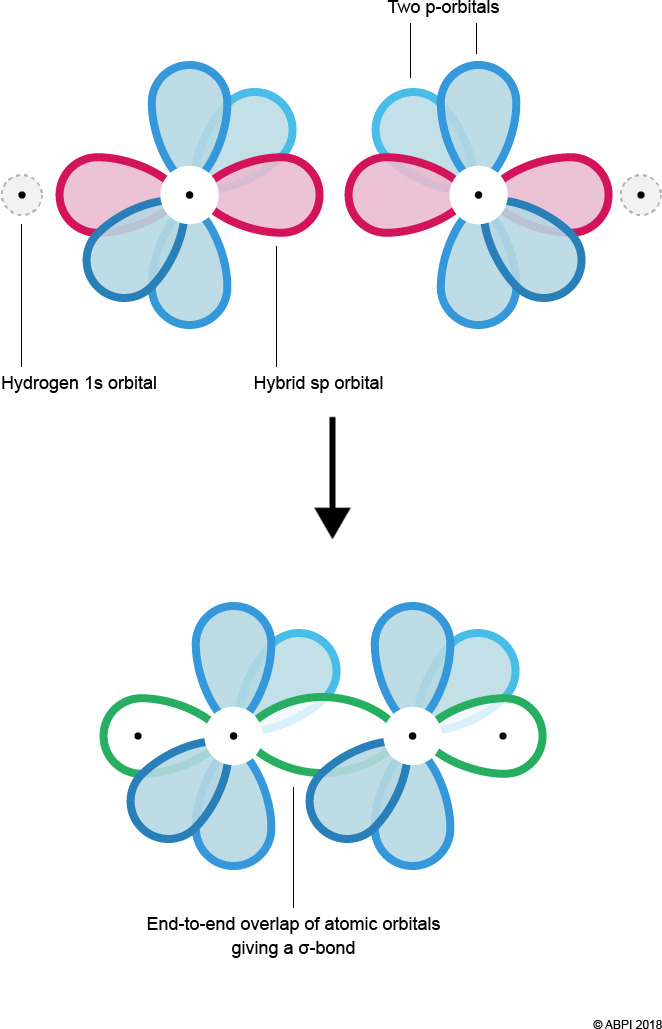
As seen before with ethene, the remaining p-orbitals cannot undergo end-to-end overlap, however they are in close enough proximity to overlap sideways. This creates 2 π-bonds.
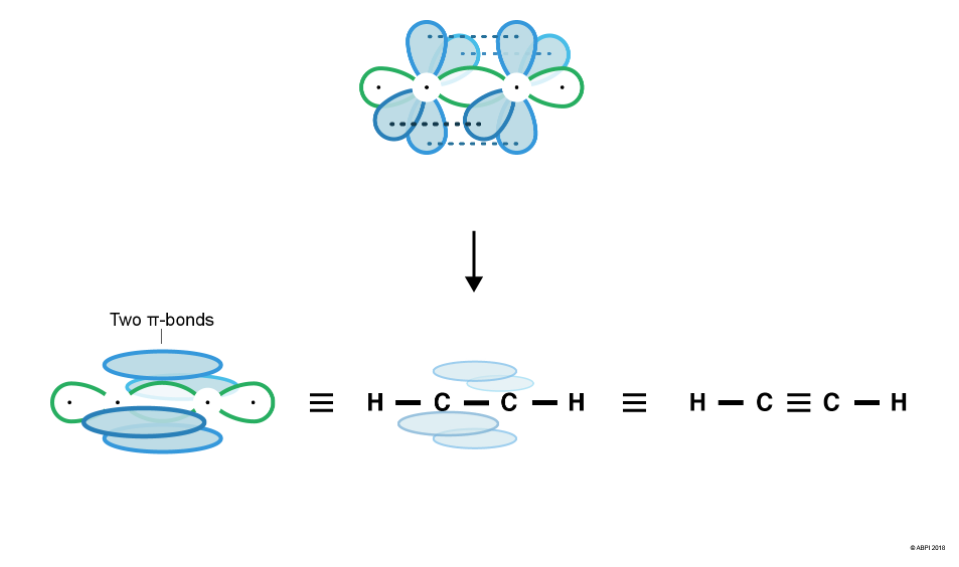
For a π-bond to exist, the p-orbitals of each Carbon must be aligned. Therefore, there is no rotation around the Carbon-Carbon triple bond as this would break the π-bond.