This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Chemistry
Chemistry
 Science
Science
 Human biology
Human biology
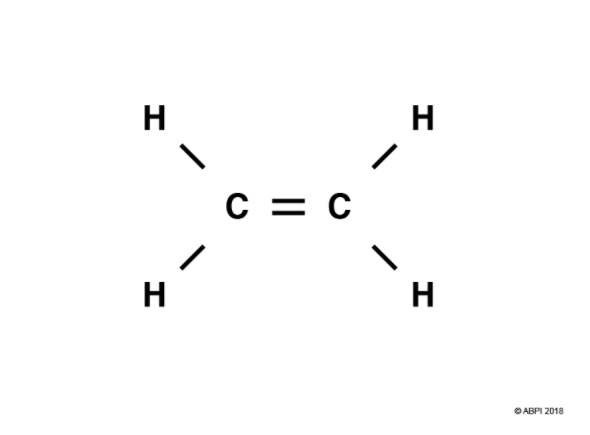
In a double bond, four electrons are shared. Let’s consider ethene, C2H4, as an example.
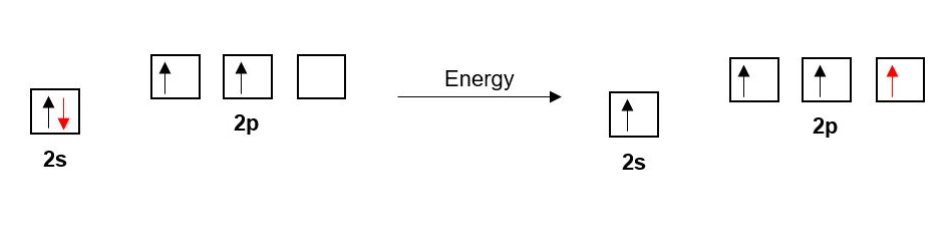
The Carbon in ethene has four bonds so as before with ethane one of Carbon’s 2s electrons is promoted to a 2p orbital.
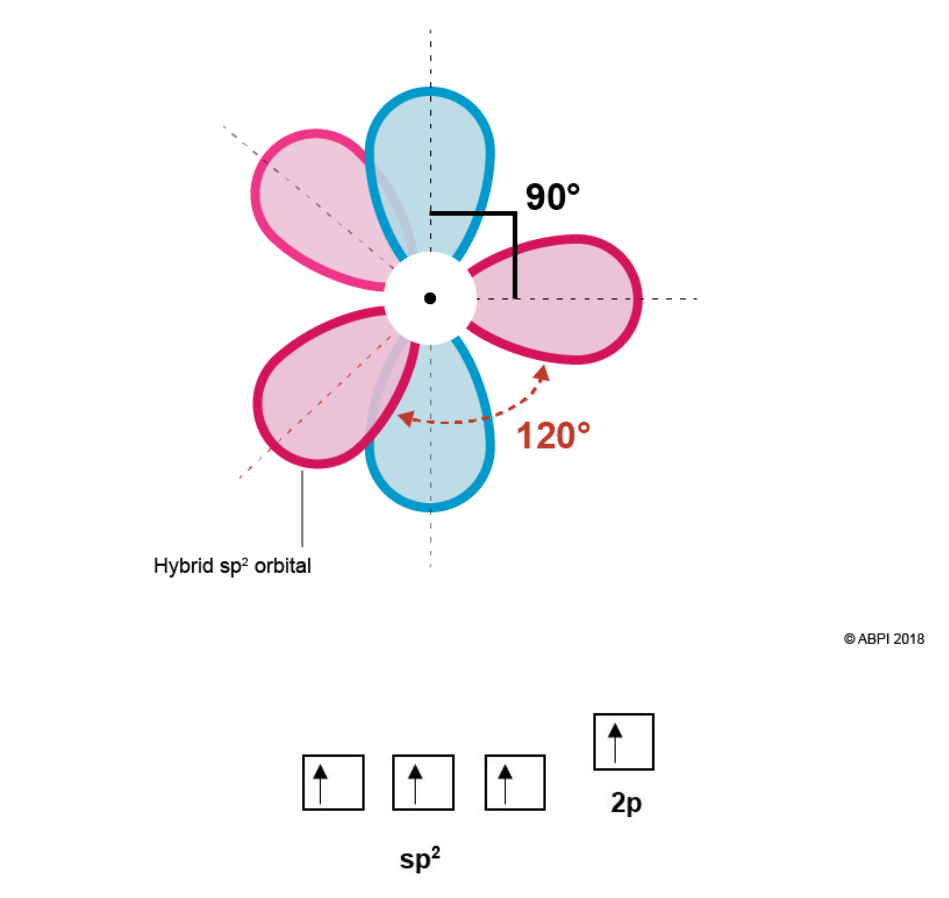
Hybridisation in ethene is somewhat different than in ethane as each Carbon in ethene is bonded to three other atoms while in ethane each Carbon is bonded to four other atoms. Therefore, only three orbitals will hybridise for ethene as opposed to all four. This will provide three equivalent hybrid sp2 orbitals and one leftover p-orbital.
Due to electron-electron repulsion the three sp2 orbitals, formed from one s-orbital and two p orbitals, rearrange themselves as far from each other as possible (120° from each other) in an equivalent trigonal planar conformation. The remaining p orbital is at a right angle (90°) right angle to the plane.
As before with ethane, overlap of Hydrogen’s 1s orbital and Carbon’s hybrid orbital (here the sp2 orbital) results in the formation of a σ-bond. In addition, the end-to-end overlap of each Carbon’s sp2 orbital also results in the formation of a σ-bond.
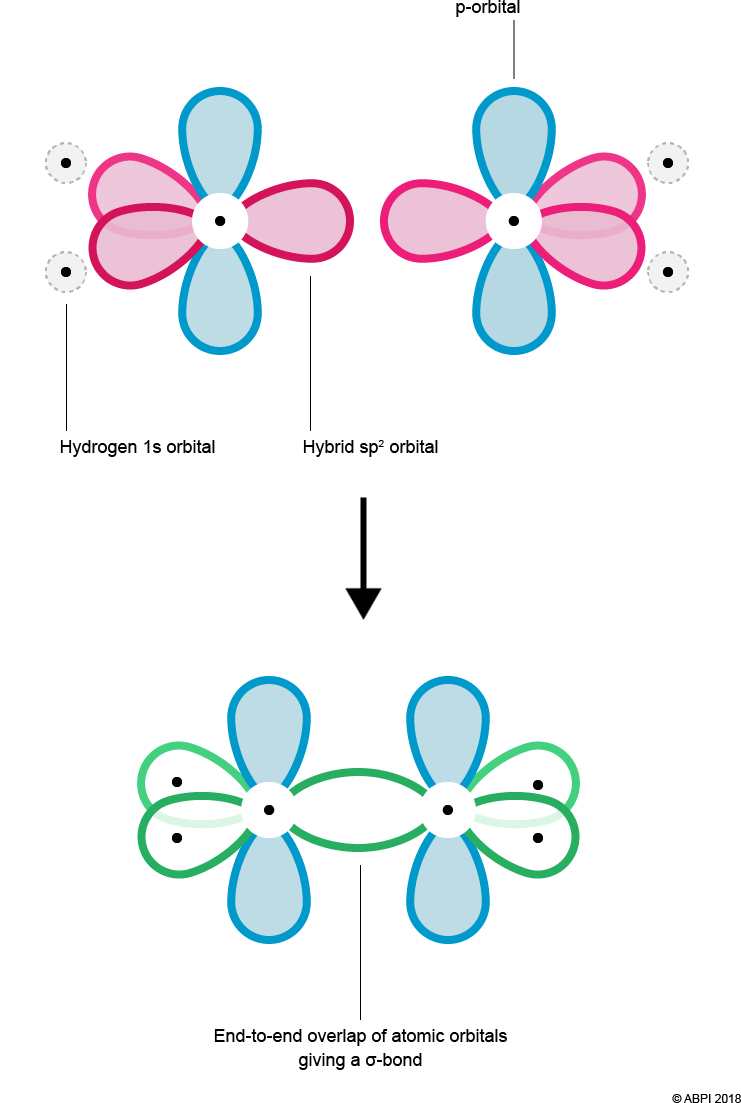
Carbon’s p orbitals are not pointing towards each other and so cannot undergo end-to-end overlap to form a σ-bond. However, they are in close enough proximity to overlap sideways. This sideways overlap creates a molecular orbital known as a π-bond.
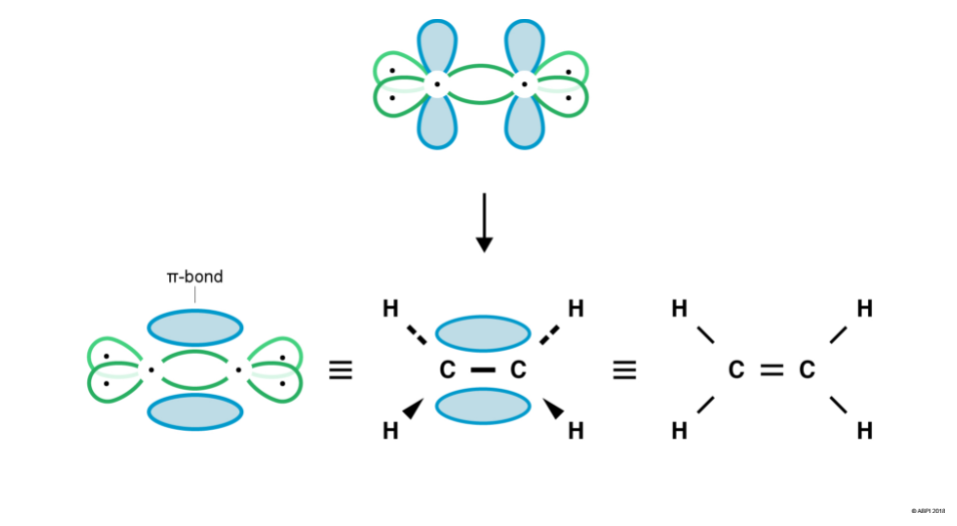
A π-bond exists above and below the plane of the σ-bond, with a total of two electrons shared. This does not mean that one electron is above the plane and one is below the plane at any given time. The two electrons can exist anywhere in the π-bond at any given time.
For a π-bond to exist, the p orbitals of each Carbon must be aligned. Therefore, there is no rotation abound the Carbon-Carbon double bond as this would cause misalignment and break the π-bond.
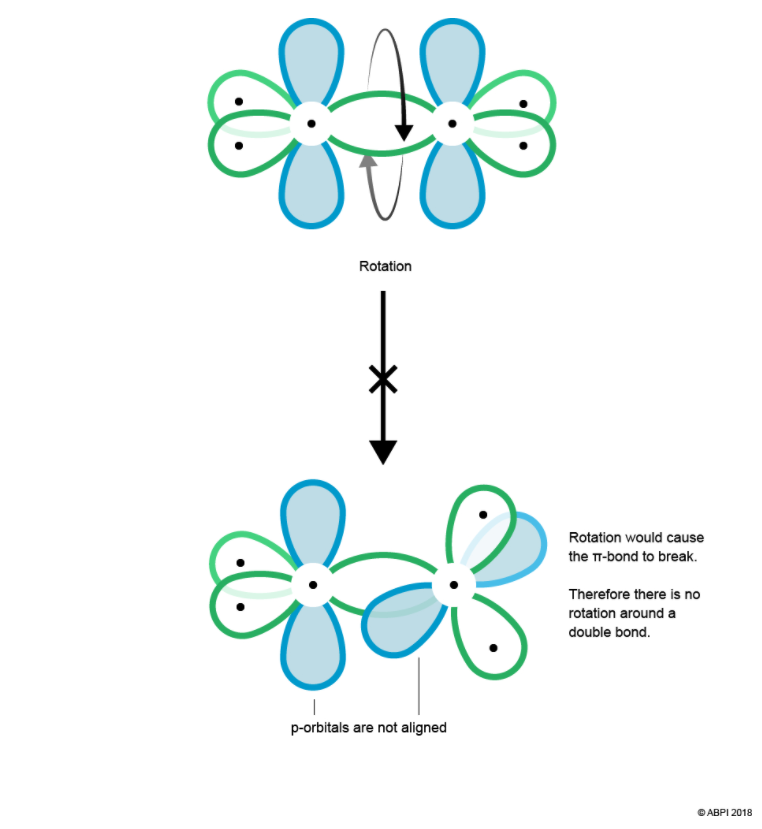
In conclusion, a double bond consists of one σ-bond and one π-bond. A π-bond is weaker than a σ-bond as it is further from the control of the nuclei. As such, π-bonds play an important role in chemical reactivity as their electron density is easily polarised.