This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Chemistry
Chemistry
 Science
Science
 Human biology
Human biology
For Carbon-Carbon bond formation, consider ethane, C2H6. As before, each Carbon atom undergoes hybridisation giving four sp3 orbitals for the formation of four bonds.
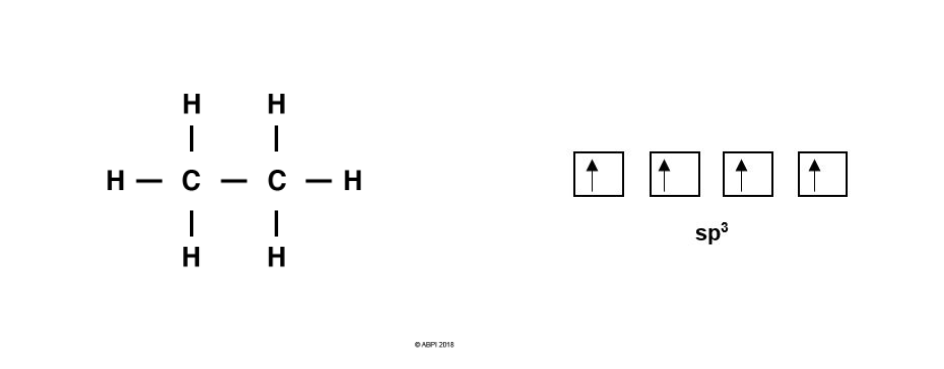
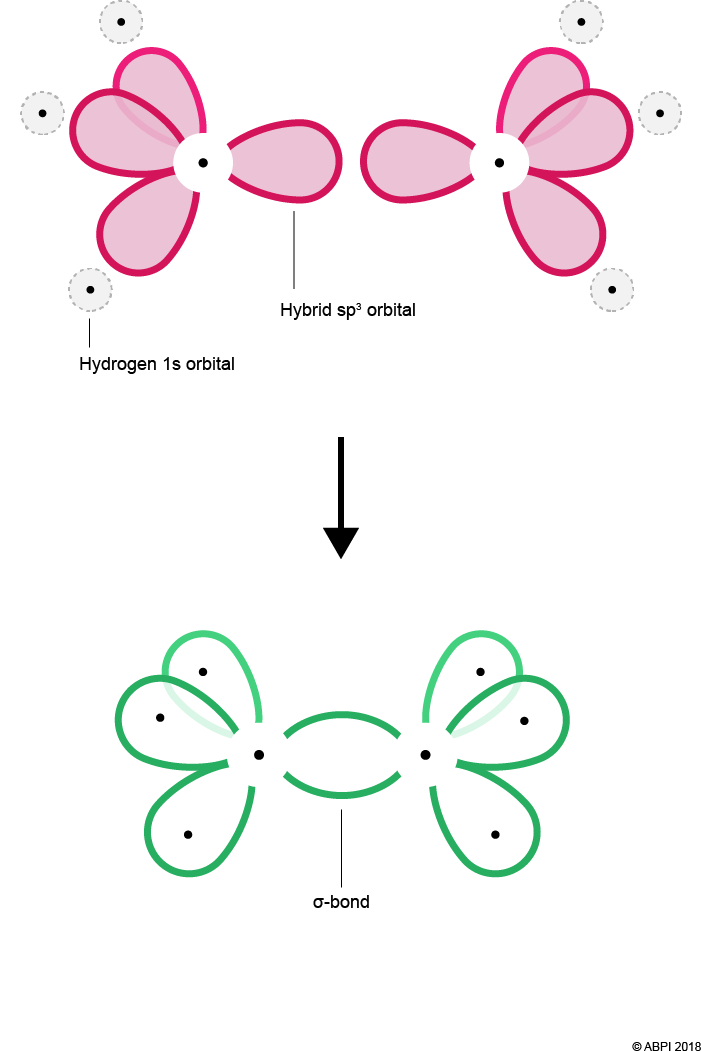
Each Carbon atom bonds to three Hydrogens. As before, each Hydrogen 1s orbital merges with a Carbon sp3 orbital to give a σ-bond. As there are three Hydrogens to bond with each Carbon, one of the four sp3 orbitals remains for each Carbon.
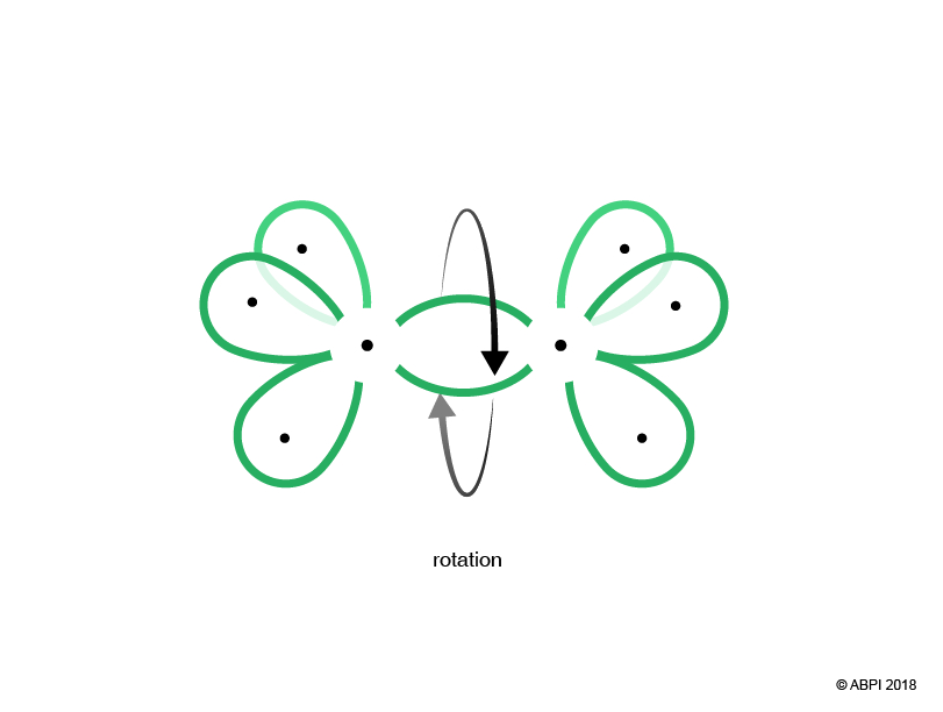
The remaining hybrid orbitals, one on each Carbon, undergo end-to-end overlap with each other to give a new molecular bonding orbital. Again, this merging produces a σ-bond with two electrons shared within it.
It is important to note that there is free rotation around σ-bonds as it does not compromise the end-to-end overlap required for bonding.