This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Chemistry
Chemistry
 Science
Science
 Human biology
Human biology
For Chromium, you would expect Cr: [Ar] 3d44s2, however it is actually Cr: [Ar] 3d54s1. Similarly for Copper one would expect Cu: [Ar] 3d94s2, however it is actually Cu: [Ar] 3d104s1.
Some people try to explain the break in pattern by claiming that half-filled or completely-filled d orbitals are more stable than a completely-filled s-orbital. For the purposes of A-level examinations this is a satisfactory explanation, however this is not actually the case! Continue to the Stretch & Challenge section if you wish to learn more.
Continuing from Chromium:
Cr: [Ar] 3d54s1
Mn: [Ar] 3d54s2
Fe: [Ar] 3d64s2
Co: [Ar] 3d74s2
Ni: [Ar] 3d84s2
Cu: [Ar] 3d104s1
Zn: [Ar] 3d104s2
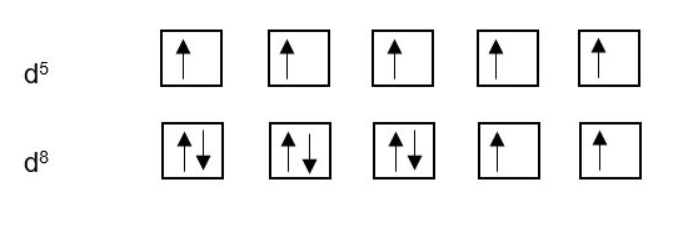
Remember, electrons will fill available orbitals singly (spin aligned) before pairing up (spin paired). This is illustrated for d5 and d8: