This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Chemistry
Chemistry
 Science
Science
 Human biology
Human biology
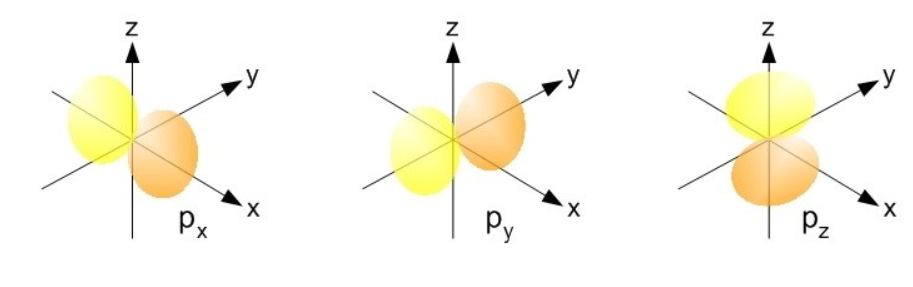
There are different types of orbitals with different shapes. Besides the s orbital, there are three dumbbell shaped p orbitals, each equal or degenerate in energy, px, py and pz. Each of these orbitals are aligned on their respective axis:
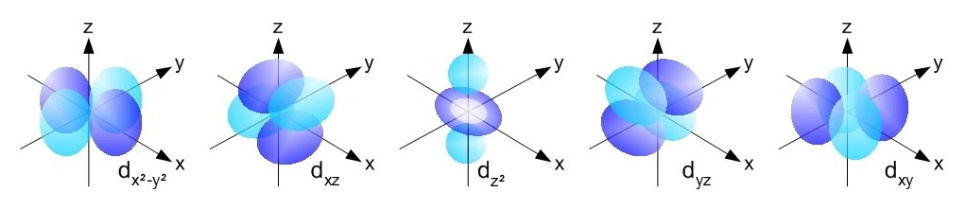
There are five degenerate d orbitals, dxy,dxz,dyz,dx2-y2 and dz2. Their shape varies from that of s and p orbitals. Note also that dz2 has a somewhat difference shape than the rest of the d orbitals.
There are seven degenerate f orbitals. Do not worry about the shapes of these orbitals. It is unlikely that you will be expected to know them as Chemistry with these orbitals is beyond the scope of A-level.
Each orbital can possess up to two electrons. Therefore the three p orbitals can possess a total of 6 electrons. When 2 electrons occupy the same orbital their spins are opposite to each other (we call these spin up and spin down) but more on that later.
To summarise:
| Orbital Name | Orbital Abbreviation | Number of Degenerate Orbitals | Total Number of Electrons |
| Spherical | s orbital | 1 orbital | 1 × 2 e- = 2 electrons |
| Principal | p orbital | 3 orbitals | 3 × 2 e- = 6 electrons |
| Diffuse | d orbital | 5 orbitals | 5 × 2 e- = 10 electrons |
| Fundamental | f orbital | 7 orbitals | 7 × 2 e- = 14 electrons |
Not all four types of orbitals are found at each energy level. In the next section you’ll learn which orbitals are found at which energy levels and how electrons are organised within them.