This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
To develop a new medicine it is important to understand how the human body works and how it is affected by a particular disease. A successful medicine treats hundreds of thousands or even millions of patients. It also makes money for the pharmaceutical company which in turn enables them to carry out research into more new medicines to treat more diseases. For any medicine to be successful it must be:
Research into a new medicine has to make sure that all these conditions are met. This is why it takes a very long time - up to 12 years - and a great deal of money - up to around £550 million - to bring a new medicine into the doctor's surgery.
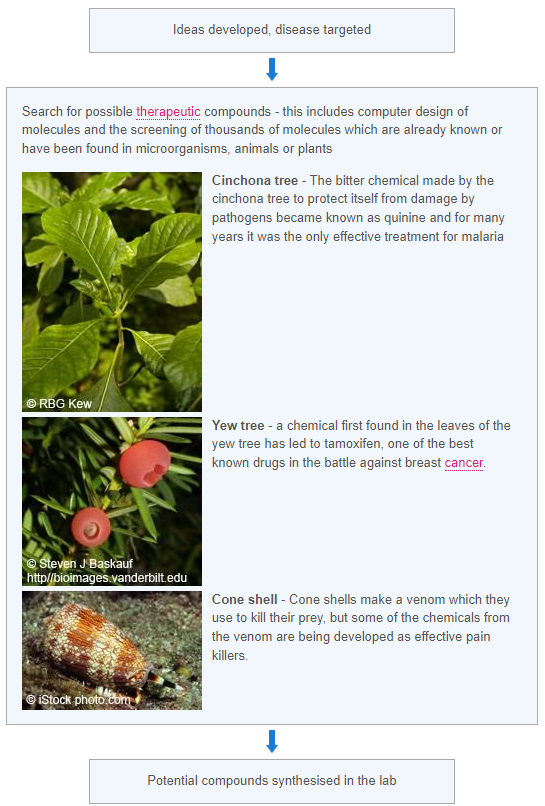
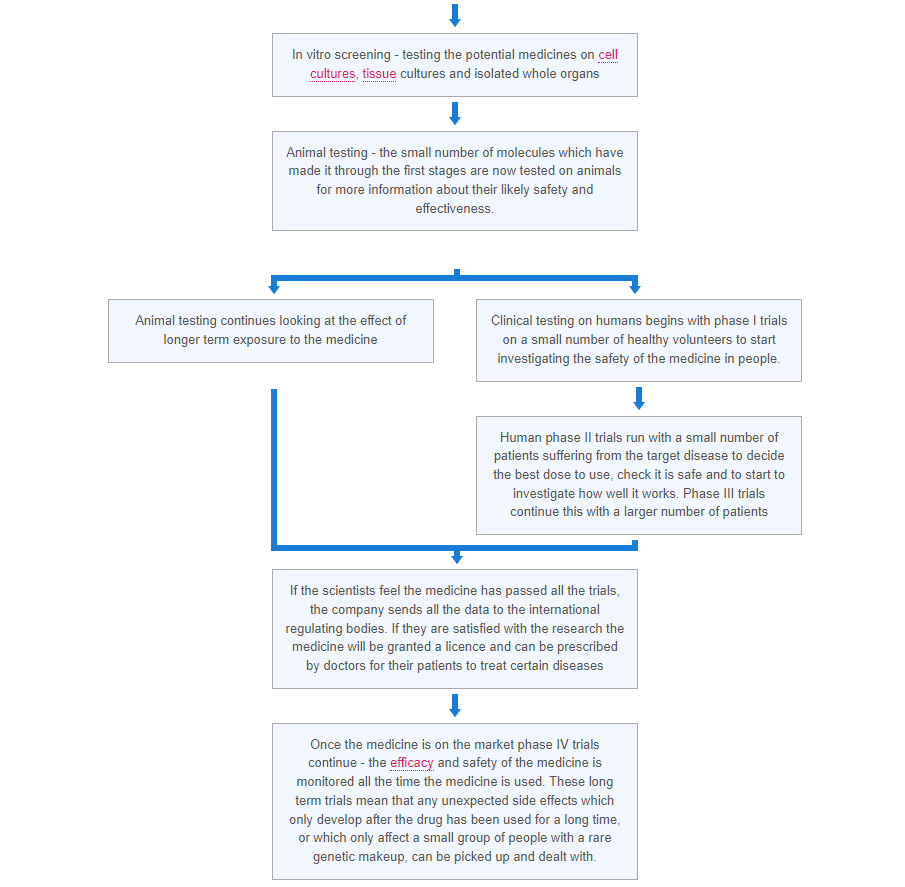
When new medical treatments are being tested there are a number of ways the trials can be run. These include:

© iStock photo.com