This topic takes on average 30 minutes to read.
There are a number of interactive features in this resource:
 Science
Science
 Chemistry
Chemistry
 Biology
Biology
Acid Rain:
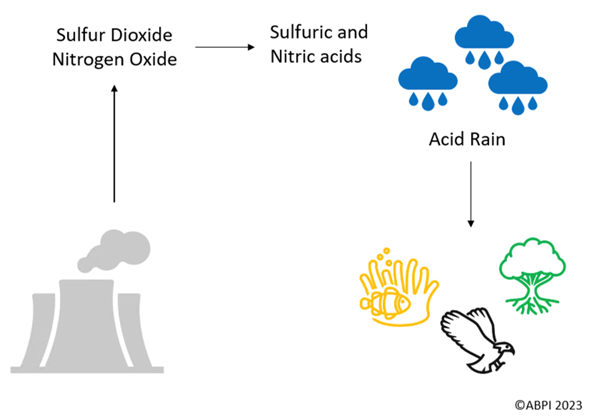
Acid rain is a type of precipitation which has an acidic pH, and this is caused by burning fossil fuels. Burning fossil fuels adds sulfur dioxide and nitrogen oxide into the atmosphere, which can react with water and oxygen to form sulfuric and nitric acids which contaminate the rain.
When acid rain falls, the rain is added into the water system.
The consequence of this is that the ocean is becoming too acidic for aquatic life, which also affects the food chain. For example, this affects birds who eat fish, as they have reduced food availability. In fact, the pH of the ocean is around 8.1 but it used to be around 8.2. Though this seems like a small change, it is big enough of a difference for aquatic life to be affected.
Acidic oceans also place threat on coral reefs.
Acid rain can be absorbed by the ground too, removing nutrients from the soil which plants and trees need to grow.
Carbon Dioxide and Ocean Acidification:
Increased CO2 emissions can also make the ocean more acidic, since CO2 dissolved in sea water becomes carbonic acid (H2CO3). This produces the same effect as acid rain, with damage to aquatic life and subsequent food chain disturbances being likely.