This topic takes on average 90 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Physics
Physics
 Science
Science
Fossil fuels are examples of non-renewable resources, and these include coal, oil and gas. They are made over millions of years and the rate at which humans are using them means that they will run out soon; this means they are finite resources.
Useful fuels are alkanes, which are saturated hydrocarbons. This means that they contain only hydrogen and carbon, and that they contain no carbon-to-carbon double bonds. Alkanes are also known as a homologous series, meaning that they all have similar properties and react in a similar way.
The diagram below shows the first four alkanes in the series, meaning that they are the alkanes which have the four smallest hydrocarbon chains.
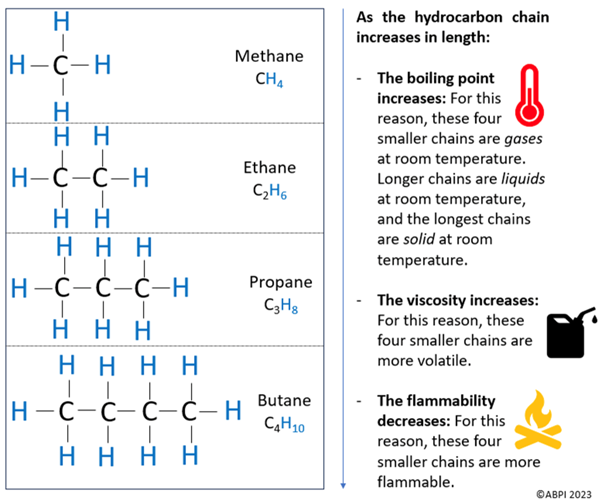
An alkane can be a hydrocarbon chain of any length, and this can be remembered using the general formula CnH2n+2. As an example, if there were 18 carbons, n is 18. Therefore, there are 2n+2 hydrogens, which is 38. So, the formula would be C18H38.
Crude oil is mostly a mixture of alkanes and these need to be separated to be useful. This is done using fractional distillation, which separates the hydrocarbon chains based on their size due to their different boiling points. Longer chains have higher boiling points than shorter ones, which means that chains of similar sizes can be separated into different fractions.
As the chains are different lengths, they have different properties. This means that the fractions are all used for different things.
The diagram below shows how fractional distillation is performed.
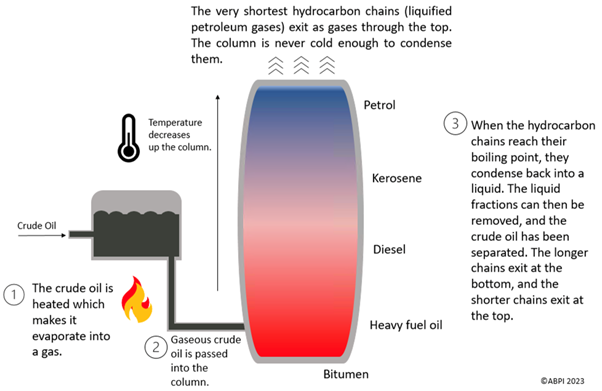
Once separated, the crude oil can be used as fossil fuels such as petrol, diesel, kerosene, heavy fuel or liquefied petroleum gas. The products can also be used as feedstock for the petrochemical industry, which means that they can be used to make solvents, lubricants, polymers and detergents.
The fossil fuels which are extracted from crude oil via fractional distillation are combusted to generate energy.