This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
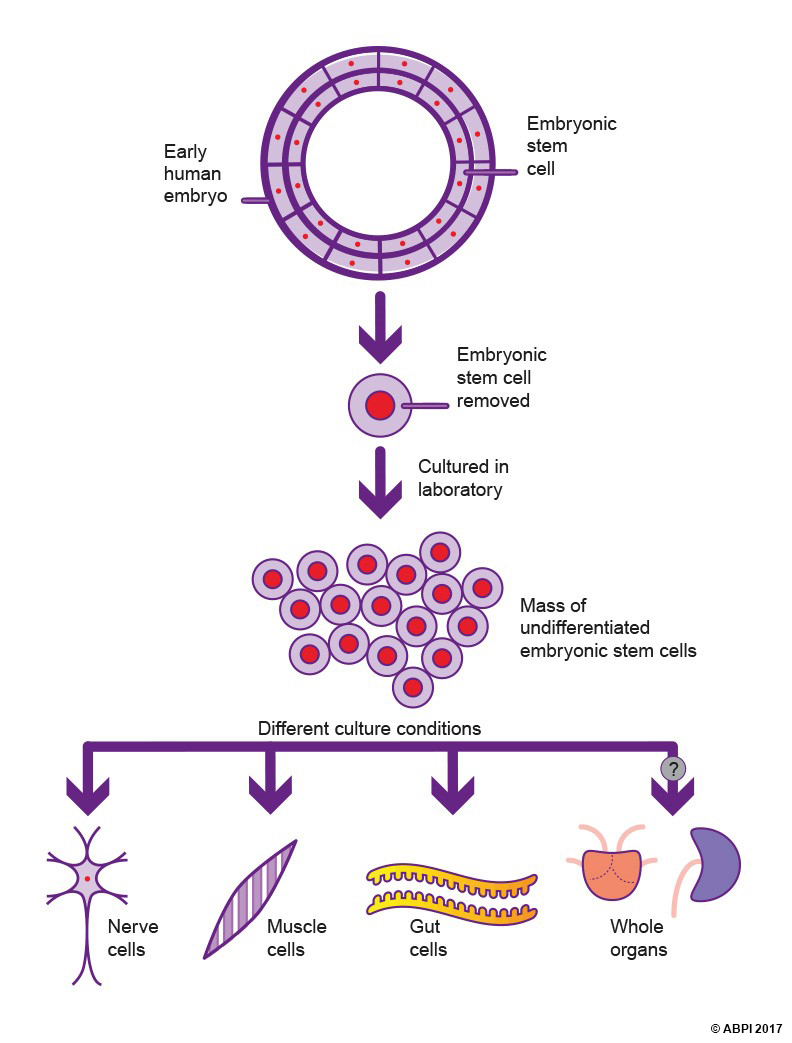
The stem cells of an early embryo such as a blastocyst have undergone very little differentiation. The genes which are needed to form the placental tissues and the amniotic membranes have been switched off, but not much else. So they would seem to be the ideal candidates for producing tissues and organs as required for patients with needs ranging from new insulin-producing cells to entire new organs.
Embryonic stem cells (ESCs) can be harvested (which destroys the embryo) and grown in culture in special conditions in the lab to produce large numbers of undifferentiated cells, which can then be used for medical purposes. By changing the conditions, the cells are living in, scientists are able to make the stem cells differentiate to produce specialised cells with many different functions. This process is usually ‘trial and error’, as no one is completely sure what conditions make cells differentiate into specific cell types. However, with experience scientists are becoming more expert at directing ESCs in the direction they want them to go.
Embryonic stem cells can only be generated from early human embryos, and opinion is divided as to whether work using ESCs is ethically viable. The decision whether to use them in research involves deciding whether the right of a potential future human to life is greater than the need to prevent suffering and cure diseases in existing people.
For this reason, the use of embryonic stem cells for research in the UK is strictly controlled. Any organisation holding a human tissue sample, such as a stem cell culture, must hold a licence from the Human Tissue Authority (HTA). Human embryonic stem cell research can only be carried out in the UK by scientists holding a licence from the Human Fertilisation and Embryology Authority (HFEA). Licences are only granted to use embryos which have been created from eggs fertilised outside of the body. In most cases, these are embryos created during infertility treatment but ultimately not used, and donated with the full consent of the parents. These licences are only granted for specific reasons including research which will result in increased knowledge about the development of embryos, the causes of miscarriages, more effective contraception or to promote infertility treatments. Other reasons include research into the causes of serious disease, to test possible diagnostic tests for gene or chromosome diseases or to learn more about or develop treatments for serious disease. In all cases a licence is only granted if the HFEA is satisfied the use of embryos and embryonic stem cells is necessary for the research.