This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Science
Science
 Biology
Biology
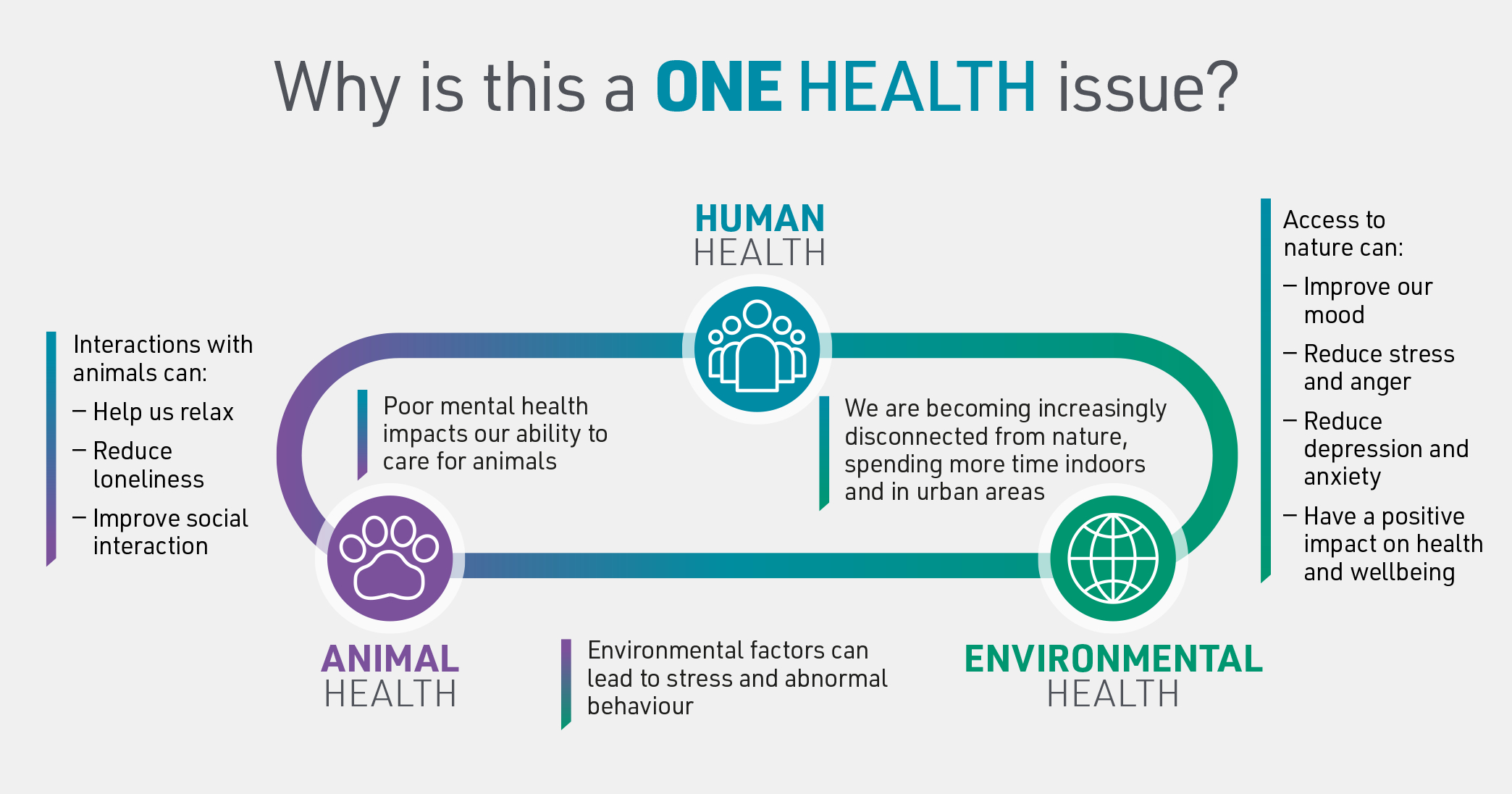
The health and wellbeing of people, animals, and the planet are interconnected, meaning that they depend on and react to each other. One Health is an idea which aims to increase collaboration between professionals who protect the health of humans, animals, and the environment. By working together, we gain expertise and knowledge which will help us to react to future challenges.
EMERGING INFECTIOUS DISEASES: Infections that have recently appeared in a population or which are spreading rapidly outside their range.
ANTIMICROBIAL RESISTANCE: Antimicrobial resistance is defined as the ability of a micro-organism to grow or survive in the presence of an antimicrobial at a concentration that is usually sufficient to inhibit or kill micro-organisms of the same species.
ZOONOSES: Diseases that can spread between animals and humans.
‘Emerging infectious diseases’ and ‘antimicrobial resistance’ are two important, global issues. Since they are connected to human health, animal health, and the environment, these topics benefit from the collaborative approach of the One Health initiative.
Over the last three decades, nearly 75 percent of all emerging human infectious diseases originated in animals1. To prevent zoonoses (diseases that can spread between animals and humans e.g. influenza or “flu”, and rabies) and control antimicrobial resistance, it is important that information is shared between professionals in a range of sectors (public health, animal health, plant health, the environment), as one sector alone cannot eliminate or handle the global challenges without interaction with the other sectors involved.
VACCINES/VACCINATION: Vaccines are important in protecting humans and animals from disease. A vaccine contains a weakened or dead form of a pathogen, its toxins or surface proteins. The immune system reacts to the vaccine as if it contained a functioning, disease-causing microorganism. The immune system is ‘trained’ to fight off the disease so that if the person or animal encounters it in future, an infection can be prevented.
The risk of infection by zoonoses grows as our population increases, and people live in ever closer contact with animals. To prevent these types of infections, the veterinary surgeons who provide animal healthcare services to people who look after companion animals (e.g. dogs and cats), as well as farm animals (e.g. pigs and cows), can use vaccinations and other medicines as appropriate to help prevent zoonotic diseases. Vaccination has long been an effective way to reduce disease burden in pets and farm animals, and is a key tool in maintaining animal health and welfare. Vaccines play an increasingly vital role in preventative health and disease control programmes in animals. Other measures, such as appropriate hygiene precautions being taken by farmers and pet owners also plays an essential role in reducing spread of zoonotic disease.
Animals can be vaccinated to prevent the spread of infectious diseases such as rabies where the use of vaccines protects both human and animal health; the animal is protected from the disease by the use of the vaccine, while humans are protected because if they encounter the animal (e.g. a dog) if the dog is protected against rabies then human health is also protected as the dog will not become infected and subsequently infect the person.
In relation to antimicrobial resistance key medicines such as antibiotics must be prescribed by a veterinary surgeon, just as antibiotics in human medicine are only available with a prescription. By ensuring that vets play a key gate keeping role and control access to antibiotics, this measure protects against misuse in animal health. Prevention of misuse of antibiotics in both human and animal health helps to prevent antimicrobial resistance leading to treatment failures in both human and animal health.