This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Human biology
Human biology
 Physical education
Physical education
Each cell in your body is effectively a bag of chemicals contained in a partially permeable membrane. Water and small particles such as some mineral ions can pass in and out of the membrane freely down concentration gradients. However, many chemicals such as glucose, amino acids and proteins cannot pass through the membrane at all - they are too big.
Liquid found inside cells is called intracellular fluid, whereas that found outside of cells is called extracellular fluid (ECF). The cells of your body are bathed in tissue fluid. This fluid, together with blood plasma, make up 97% of the ECF. The remaining 3% is lymph. Tissue fluid, or interstitial fluid, is a solution of salts, sugars and other chemicals in water. It is important that the concentration of dissolved substances in the tissue fluid is the same as the concentration of the cell contents in the cytoplasm (an isotonic solution). If the solution outside the cell is more concentrated than the cell contents (a hypertonic solution), water will move out of the cell by osmosis. If the solution outside the cell is less concentrated that the cell contents (a hypotonic solution), then water will move into the cell by osmosis.
You can make model cells using Visking tubing (a partially permeable membrane) and observe the effects. They help demonstrate why it is important that the water balance of the tissue fluid of the body is kept constant.
Effect of osmosis on red blood cells
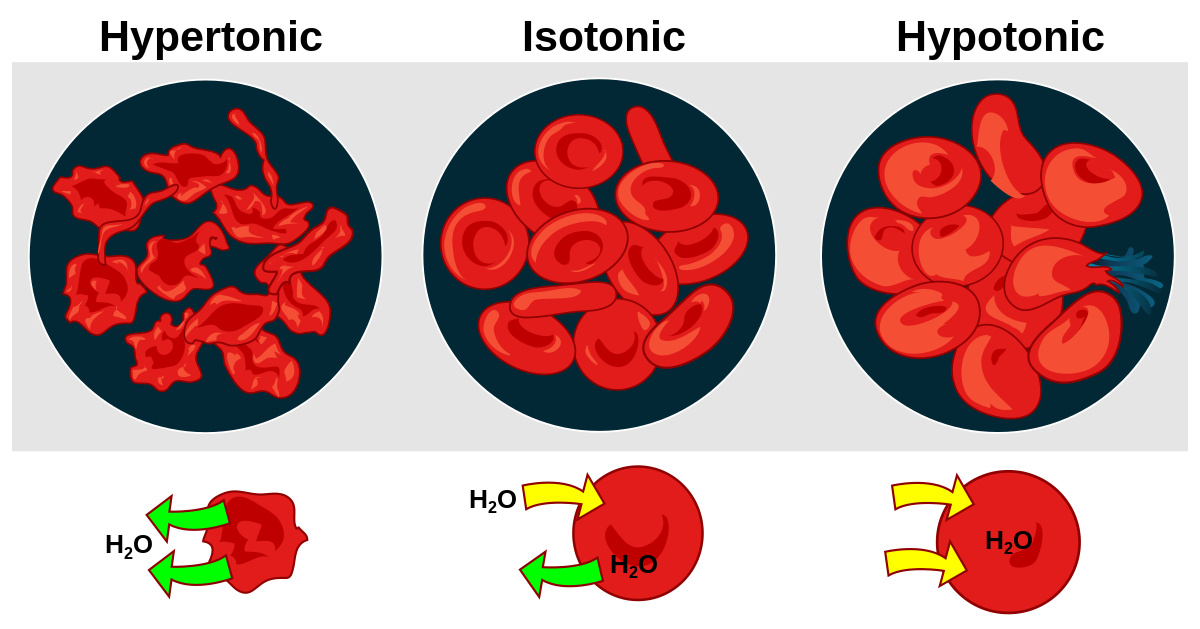
If the surrounding fluid becomes more concentrated (hypertonic) than the red blood cell contents, they lose water by osmosis and cannot function.
If the surrounding fluid is instead less concentrated (hypotonic), the red blood cell gains water by osmosis, and could even burst.
See below for an animation which shows you the effect of water balance problems on the red blood cells which carry oxygen around your body.