This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 PSHE / Citizenship studies
PSHE / Citizenship studies
Cloning is still very much a research technique rather than a commercial technique. Therefore cloned animals are often produced to test the possibilities of cloning and improve the methods used rather than for an established use.
There are several areas in which cloning can be, and is, used by scientists: this includes cloning genetically engineered livestock for a medical use, and cloning useful working animals such as sniffer and guide dogs. In both these situations, however, the few clones that have been produced were created to see if their creation was possible, rather than to provide a specific product or service.
Livestock have also been cloned for commercial reasons, particularly in the US, although this is banned in Europe. The cloned animals were created to provide a special service as a breeding parent to create offspring that could enter the food chain.
Since the birth of Dolly, more sheep cloned from adults have been born. Polly and Molly were transgenic sheep created with a specific use in mind. They were the result of a process in which a human gene had been introduced into somatic cell nuclei. These nuclei were then transferred into unfertilised enucleated eggs which were implanted into surrogate mother sheep. Polly and Molly produced milk containing human blood clotting factor IX, which is deficient in patients with haemophilia B. Haemophilia B patients do not stop bleeding when they cut themselves; this can be treated by injecting factor IX into their blood so that their blood can start to clot around the wound. Factor IX is usually extracted from human blood but extracting it from milk would be much cheaper and there would be less chance of a serious infection being passed to the patient. This has not yet led to commercial flocks of sheep producing factor IX in their milk.
Cloning a transgenic organism ensures that the introduced gene, and therefore the useful protein it produced, will definitely be inherited – which wouldn’t be so certain if a transgenic organism reproduced in the normal way. Inserting a gene requires a lot of time and effort (and therefore also a lot of money) and so it is beneficial if the process doesn’t need to be repeated over and over again.
In 2009 the first drug from transgenic animals was approved in the USA. This was human anti-thrombin-α produced by genetically modified cloned goats. In 2014 another protein medicine produced by transgenic animals was approved in the USA. This time it was a protein used to treat patients with hereditary angioedema. The protein is produced in the milk of transgenic cloned rabbits.
Cloning transgenic organisms isn’t just limited to sheep. Many people on organ waiting lists die each year because there are far more people needing an organ than organ donors, so using organs from another source provides the opportunity to reduce a lot of suffering. One way of doing this is xenotransplantation - using organs from another species. Pigs are a popular choice in xenotransplantation research: pig organs are a similar size to humans’, pigs reproduce quickly and have many piglets each litter, and are easy to rear in a disease-free environment.
Unfortunately, the antigens on the surface of their cells are recognised as being foreign by the human immune system which therefore attempts to destroy them. Transgenic pigs have already been produced that lack certain immune response-producing antigens. Once pigs have been produced that lack all the antigens that create an immune reaction in humans, cloning them can easily increase the numbers of pigs suitable to be used as organ sources and therefore also the number of human lives that can be saved. New technologies such as CRISPR-Cas9 will make the process of editing the pig genome to remove the antigens which trigger a response in humans much faster and more accurate. Already scientists can edit a number of genes at the same time.
There is a danger, however, that transplanting an organ from another species into a human may introduce a new disease into humans: pig viruses have been shown to infect cultured human cells. The risk of this happening is minimised by keeping the pigs in isolation in sterile conditions - but these conditions would be unnatural and raise questions about animal welfare. There is an increased interest in xenotransplantation, both because new technologies make it more feasible, and because stem cell technologies are taking a long time to develop. But there is still a long way to go before cloned animals can provide us with the medicines or the spare organs which we need.

Reproductive human cloning is currently illegal, but in December 2016 the final hurdle was overcome in the UK to allow the development of a new form of treatment known as mitochondrial donation. This is also popularly known as three-person IVF. What does this mean – and why is it necessary?
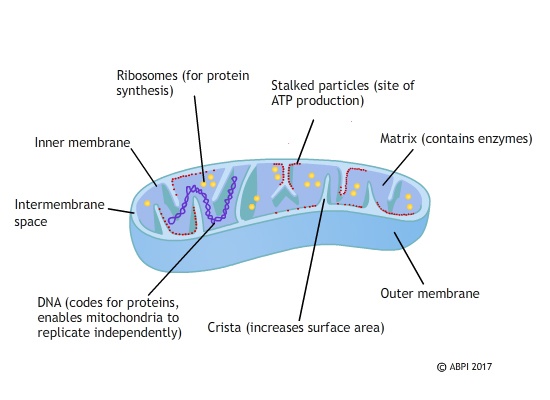
The mitochondria are the organelles where the reactions of aerobic respiration take place. The reactions of glycolysis and Krebs cycle are the main source of ATP in the body, and ATP is needed for almost every other reaction in the cells to take place. If the mitochondria are faulty it is almost always fatal either before or after birth. The mitochondria are inherited only from the mother and passed on in the cytoplasm of the egg. They have their own set of genes which code for the proteins involved in producing ATP – if these genes are damaged, the patient can develop a serious untreatable disease. If these mitochondria could be replaced with healthy ones, the disorder could be prevented.
Mitochondrial donation is a new technique designed to prevent mitochondrial diseases being passed on from mothers to their children. Scientists have developed a technique where an egg from the affected mother (containing diseased mitochondria) is fertilised with the father’s sperm (which doesn’t contribute any mitochondria) and then the resulting diploid nucleus is removed. This nucleus is then transferred to a donated enucleated egg with healthy mitochondria from a different woman – essentially embryonic cell nuclear transfer.
Any baby born would not be a clone because it would not be a genetic copy of a pre-existing organism – but as this method involves many techniques used in reproductive cloning it has been subject to a great deal of consideration and debate. There were also additional ethical issues to consider. Changes to the mitochondrial DNA will be inherited by future generations: this means that any children of a woman who was born as a result of this technique would not inherit a mitochondrial disease, but any unforeseen negative genetic effects of this treatment would also be inherited.
At the moment, any child who was born as the result of egg donation can apply for information about their donor (from whom they have received half their genetic material) once they are 18 years old. Eggs used for nuclear transfer will only donate a very small amount of genetic material to the resulting child, so the donor could remain anonymous like blood donors. Furthermore, donated eggs are in short supply; mitochondrial disease is rare so there will be relatively few couples requiring this treatment, but it is possible that there will not be enough donated eggs for all of them. You can read more about the ethical issues and technical background (including potential problems and ways to avoid them) to this treatment. They were considered by Parliament in a document you can find here. This was written to allow non-scientific MPs to understand more about prevention of mitochondrial disease.
In 2015, Parliament passed legislation making mitochondrial donation legal in the UK, and in December 2016 the Human Fertilisation and Embryology Authority (HFEA) which licences and regulates fertility treatments in the UK, approved the use of mitochondrial donation for the treatment of mitochondrial diseases. It is only a matter of time before the first healthy babies resulting from mitochondrial donation are born in the UK. However, in other parts of the world babies have already been born successfully free of mitochondrial disease, for example in 2016 a baby boy was born whose parents were treated by a US team who travelled to Mexico to carry out the procedure because it is banned in the United States. The parents had already had four miscarriages and two babies who died as a result of a form of mitochondrial disease known as Leigh Syndrome. Then in 2017 a baby girl free of mitochondrial disease was born to an affected mother in Ukraine, also as a result of three-person IVF.
So far, all the signs are positive. One concern which will only be answered with time is the question of how many faulty mitochondria will be transferred with the nucleus from the original egg. Scientists do not yet know if the percentage of faulty mitochondria will be enough to affect the health of the baby – they think not – and if the faulty mitochondria will be able to reproduce as well, or better, than the healthy ones. This is science at the cutting edge, but it has the potential to be a cure for those families affected by these rare conditions.
Many species become extinct each year – could cloning help stop this? Cloning could help increase the number of breeding couples so that more offspring could be born. Unfortunately, it can’t increase the size of the gene pool, so many people believe that cloning should not be used in these cases because it may cause so much inbreeding that genetic disorders will develop and the species becomes extinct anyway.

However, inbreeding doesn’t necessarily lead to extinction: a small herd of wild cows in Northumberland called Chillingham wild cattle have survived several centuries of inbreeding and all have incredibly similar DNA suggesting that they are descended from the same few ancestors, but despite this they are healthy.
On the other hand, the Habsburg family (who ruled several European countries) was so inbred that many genetic problems kept recurring and were worse in each successive generation, so that the last Habsburg King of Spain had learning difficulties, and a whole range of physical problems including infertility and an inability to chew or speak clearly because of inherited problems with his mouth and tongue.


Several endangered animals have already been cloned. Mouflon are a type of wild sheep found on steep mountainous slopes throughout Europe and western parts of Asia and are classified as vulnerable as there is a large risk of them becoming endangered in the wild. When a female mouflon was found dead (from natural causes) at an Italian nature preserve, researchers took a sample of some of her cells. Cloning these cells offered the opportunity to increase the future potential population size of mouflon as a new breeding female could be produced. Scientists therefore carried out SCNT using genetic material from the stored mouflon cells and domestic sheep as the egg donor and surrogate mother. The mouflon born in 2001 was the first cloned endangered mammal to survive infancy.
Unfortunately, some animals which are the last surviving member of their species die before they can be cloned. Celia, the last surviving Pyrenean ibex (a type of wild goat also known as a bucardo) died in 2000 meaning that the sub-species she belonged to became extinct. Luckily, some cells were taken from her and stored. This meant that in 2009 an attempt could be made to clone her using SCNT and domestic goat eggs. The domestic goat and the Pyrenean ibex have different pregnancy lengths, so Spanish ibex (the species most similar to the Pyrenean ibex) and Spanish ibex-domestic goat hybrids (which have a pregnancy length closer to the Spanish ibex) were used as surrogate mothers. One Pyrenean ibex was born but only lived for a few hours as she had a severe lung defect – this was possibly because freezing damaged the DNA as it was stored. The Pyrenean ibex is therefore the only sub-species to become extinct twice.

At the moment, scientists do not see cloning as a way of saving endangered species. This is partly because the technology is not yet very effective, and partly because there is little point in saving an endangered species when the causes of its extinction have not yet been tackled. However, increasingly scientists are freezing tissue samples, gametes and DNA from endangered species – for example the Frozen Ark project inspired by Professor Bryan Clarke and his wife Dr Ann Clarke. The hope is that these samples may be used in one of two ways:
to clone and revive a species when the appropriate habitat has been protected or conserved
to genetically modify other species to introduce positive survival traits.
Who knows what the future will hold?

Sniffer dogs, whilst very useful, are not easy to train: only 30% of dogs which train to be sniffer dogs are good enough to become working animals. This talent is due to both environmental and genetic reasons which is refined during the long training process. It is therefore of economic benefit to ensure that any dog with superior sniffing powers is reproduced and its special ability passed on. A seven year old golden labrador retriever called Chase with superb sniffing-powers had some skin cells extracted in his home country of Canada. These were taken to Seoul National University in South Korea where they were cloned using SCNT. Seven puppies were born which were all given the name ‘Toppy’ (for ‘tomorrow’s puppy’) and all showed enough sniffing ability to pass their training course.
Some clones have problems that affect their health when they are first born, but these problems usually disappear by the time they reach adulthood. Furthermore, these clones can breed normally and their offspring are completely normal, showing no more health problems, even at birth, than an ‘ordinary’ animal. The US Food and Drug Administration carried out a large survey and concluded in 2008 that eating meat or other products from a cloned animal or its offspring has no risks to humans. As a result, this is what many cloned animals (from both artificial twinning and reproductive SCNT) are used for: increasing the frequency of desirable traits in the gene pool of livestock.
If an animal is an excellent specimen of its breed it can be cloned so that the number of offspring with its genetic material is increased. The number of cloned animals is relatively small however, so cloned animals are much more likely to be used as breeding stock than as food themselves. In the US products from cloned animals and their offspring do not have to be labelled, but in the UK they are classed as a ‘novelty food’ and you cannot buy products from cloned animals or their offspring without knowing exactly where they come from.
